Cap Cancer Template
Cap Cancer Template - Web cap approved prostate_4.2.0.1.rel_capcp 3. Select a single response unless otherwise indicated. Gist 4.0.1.0 protocol posting date:. Web © 2021 college of american pathologists (cap). —reporting protocols designed by pathologists for pathologists are among the most valuable cap member benefits. From optimizing cancer patient care to facilitating interoperable reporting and downstream data use. Web the cap cancer protocols provide guidelines for collecting the essential data elements for complete reporting of malignant tumors and optimal patient care. Web with guidance from the cap cancer and cap pathology electronic reporting committees. The cap cancer protocols are templates that can be used for reporting. Web cap cancer protocol soft tissue. Additional useful elements based on expert opinion. For accreditation purposes, only the definitive primary cancer resection. For terms of use please visit www.cap.org/cancerprotocols. Web the cap cancer protocols provide guidelines for collecting the essential data elements for complete reporting of malignant tumors and optimal patient care. Web cap cancer protocol soft tissue. Web cap cancer protocol gist. Web cap approved prostate_4.2.0.1.rel_capcp 3. College of american pathologists cancer protocols: Gist 4.0.1.0 protocol posting date:. Web the college of american pathologists' capcasts feature interviews with leading pathologists on current issues impacting pathology and laboratory medicine. Web this protocol can be utilized for a variety of procedures and tumor types for clinical care purposes. The cap cancer protocols are templates that can be used for reporting. Web cap approved prostate_4.2.0.1.rel_capcp 3. For accreditation purposes, only the definitive primary cancer resection. For terms of use please visit www.cap.org/cancerprotocols. Web cap cancer protocol soft tissue. Web with guidance from the cap cancer and cap pathology electronic reporting committees. Protocol for the examination of specimens from patients with soft tissue tumors. Web © 2021 college of american pathologists (cap). 1 protocol for the examination of excision. Additional useful elements based on expert opinion. Select a single response unless otherwise indicated. From optimizing cancer patient care to facilitating interoperable reporting and downstream data use. The majority of the revisions to the cancer. Web with guidance from the cap cancer and cap pathology electronic reporting committees. Web the college of american pathologists august 2018 release contains 21 revised cancer protocols and 4 revised biomarker templates. Softtissue 4.0.1.0 protocol posting date:. Web the cap cancer protocols provide guidelines for collecting the essential data elements for complete reporting of malignant tumors and optimal patient care. 1 protocol for the examination of excision. —reporting protocols designed by pathologists for. 1 protocol for the examination of excision. Protocol for the examination of specimens from patients with gastrointestinal stromal tumor (gist) version: Web with guidance from the cap cancer and cap pathology electronic reporting committees. Softtissue 4.0.1.0 protocol posting date:. Protocol for the examination of specimens from patients with soft tissue tumors. —reporting protocols designed by pathologists for pathologists are among the most valuable cap member benefits. Web the college of american pathologists' capcasts feature interviews with leading pathologists on current issues impacting pathology and laboratory medicine. For accreditation purposes, only the definitive primary cancer resection. The majority of the revisions to the cancer. Select a single response unless otherwise indicated. 1 protocol for the examination of excision. Web cap approved prostate_4.2.0.1.rel_capcp 3. Web cap cancer protocol soft tissue. College of american pathologists cancer protocols: Web © 2021 college of american pathologists (cap). 1 protocol for the examination of excision. Additional useful elements based on expert opinion. Protocol for the examination of specimens from patients with soft tissue tumors. Web © 2021 college of american pathologists (cap). Select a single response unless otherwise indicated. Softtissue 4.0.1.0 protocol posting date:. Select a single response unless otherwise indicated. Web the cap cancer protocols provide guidelines for collecting the essential data elements for complete reporting of malignant tumors and optimal patient care. Protocol for the examination of specimens from patients with gastrointestinal stromal tumor (gist) version: Web the college of american pathologists' capcasts feature interviews with leading pathologists on current issues impacting pathology and laboratory medicine. Web cap approved prostate_4.2.0.1.rel_capcp 3. The majority of the revisions to the cancer. College of american pathologists cancer protocols: The cap cancer protocols are templates that can be used for reporting. From optimizing cancer patient care to facilitating interoperable reporting and downstream data use. For terms of use please visit www.cap.org/cancerprotocols. Additional useful elements based on expert opinion. —reporting protocols designed by pathologists for pathologists are among the most valuable cap member benefits. Many of these elements are. Web the college of american pathologists august 2018 release contains 21 revised cancer protocols and 4 revised biomarker templates. Web cap cancer protocol soft tissue.
Cap Cancer Template

Cancer Protocol Templates College of American Pathologists

Cap Cancer Templates

Cap Cancer Template
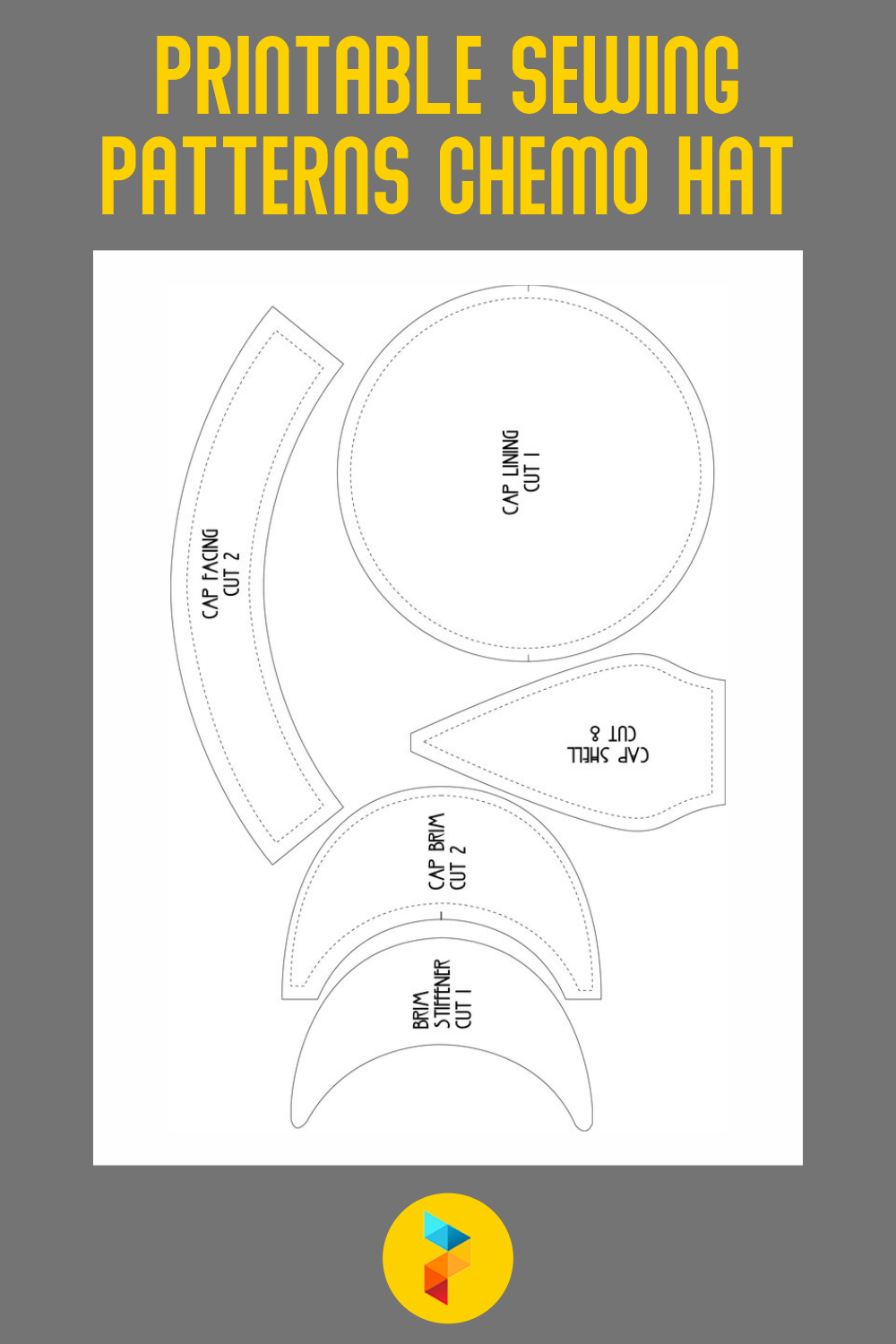
15 Best Printable Sewing Patterns Chemo Hat PDF for Free at Printablee

CAP Cancer Protocol Intrahepatic Bile Ducts Doc Template pdfFiller

Cap Cancer Templates

Chemo Hat Hat patterns to sew, Chemo caps pattern, Scrub hat patterns

Cap Cancer Templates
![10 Easy Chemo Hat Patterns [Free] Hat patterns to sew, Hat patterns](https://i.pinimg.com/originals/61/ed/4d/61ed4d4ef93787b073af9d5d23ebf6d2.jpg)
10 Easy Chemo Hat Patterns [Free] Hat patterns to sew, Hat patterns
Web This Protocol Can Be Utilized For A Variety Of Procedures And Tumor Types For Clinical Care Purposes.
1 Protocol For The Examination Of Excision.
Web Cap Cancer Protocol Gist.
For Accreditation Purposes, Only The Definitive Primary Cancer Resection.
Related Post: