Cap Protocol Templates
Cap Protocol Templates - Cancer protocols single of measure. Web view current protocols and download templates. Cap laboratory accreditation program protocol required use date: March 2022 the changes included in this current. Uterinecervix 4.1.0.0 protocol posting date: 30 day free trialfree mobile app24/7 tech supportfast, easy & secure Web cap cancer protocol soft tissue. Ready more about the kappen notice regarding unit of measurement in the cap. Web the cap cancer protocols contain guidance and standardized clinical content that support best practices in cancer patient care. —reporting protocols designed by pathologists for pathologists are among the most valuable cap member benefits. Web the college of american pathologists' (cap) “protocol for the examination of resection specimens from patients with primary carcinoma of the colon and rectum” must be. Softtissue 4.0.1.0 protocol posting date:. For terms of use please visit www.cap.org/cancerprotocols. Protocol for the examination of specimens from patients with carcinoma and carcinosarcoma of the endometrium. March 2022 the changes included in this. March 2022 the changes included in this current. Uterinecervix 4.1.0.0 protocol posting date: Web view current protocols and download templates. Protocol for the examination of specimens from patients with soft tissue tumors. Ready more about the kappen notice regarding unit of measurement in the cap. College of american pathologists cancer protocols: Protocol for the examination of specimens from patients with carcinoma and carcinosarcoma of the endometrium. Protocol for the examination of specimens from patients with soft tissue tumors. Web view current protocols and download templates. For terms of use please visit www.cap.org/cancerprotocols. Softtissue 4.0.1.0 protocol posting date:. Web protocol for the examination of specimens from patients with primary carcinoma of the uterine cervix. From optimizing cancer patient care to facilitating interoperable reporting and downstream data use. 30 day free trialfree mobile app24/7 tech supportfast, easy & secure March 2022 the changes included in this current. From optimizing cancer patient care to facilitating interoperable reporting and downstream data use. Web the college of american pathologists' (cap) “protocol for the examination of resection specimens from patients with primary carcinoma of the colon and rectum” must be. 30 day free trialfree mobile app24/7 tech supportfast, easy & secure Cap laboratory accreditation program protocol required use date: June 2021. For terms of use please visit www.cap.org/cancerprotocols. March 2022 the changes included in this current. Web cap cancer protocol soft tissue. Web the cap cancer protocols contain guidance and standardized clinical content that support best practices in cancer patient care. Protocol for the examination of specimens from patients with carcinoma and carcinosarcoma of the endometrium. Ready more about the kappen notice regarding unit of measurement in the cap. Web protocol for the examination of specimens from patients with carcinoma of the stomach. Uterinecervix 4.1.0.0 protocol posting date: Cap laboratory accreditation program protocol required use date: Web © 2021 college of american pathologists (cap). June 2021 this biomarker template is not required for accreditation purposes but may be used to facilitate compliance with cap. Web cap cancer protocol soft tissue. Protocol for the examination of specimens from patients with soft tissue tumors. Protocol for the examination of radical. Softtissue 4.0.1.0 protocol posting date:. Cap laboratory accreditation program protocol required use date: Stomach 4.1.0.0 protocol posting date: Web protocol for the examination of specimens from patients with carcinoma of the stomach. Cap laboratory accreditation program protocol required use date: Web © 2021 college of american pathologists (cap). Web © 2021 college of american pathologists (cap). Protocol for the examination of radical. 30 day free trialfree mobile app24/7 tech supportfast, easy & secure —reporting protocols designed by pathologists for pathologists are among the most valuable cap member benefits. College of american pathologists cancer protocols: Cancer protocols single of measure. Web on march 22, 2023, the college of american pathologists released updates to 10 cap cancer protocols. Softtissue 4.0.1.0 protocol posting date:. Web cap cancer protocol soft tissue. Stomach 4.1.0.0 protocol posting date: —reporting protocols designed by pathologists for pathologists are among the most valuable cap member benefits. Web the college of american pathologists' (cap) “protocol for the examination of resection specimens from patients with primary carcinoma of the colon and rectum” must be. March 2022 the changes included in this current. March 2022 the changes included in this current. Web protocol for the examination of specimens from patients with carcinoma of the stomach. Protocol for the examination of specimens from patients with soft tissue tumors. 30 day free trialfree mobile app24/7 tech supportfast, easy & secure Web view current protocols and download templates. Protocol for the examination of radical. Cap laboratory accreditation program protocol required use date: From optimizing cancer patient care to facilitating interoperable reporting and downstream data use.
Download our free cap table template Vestd
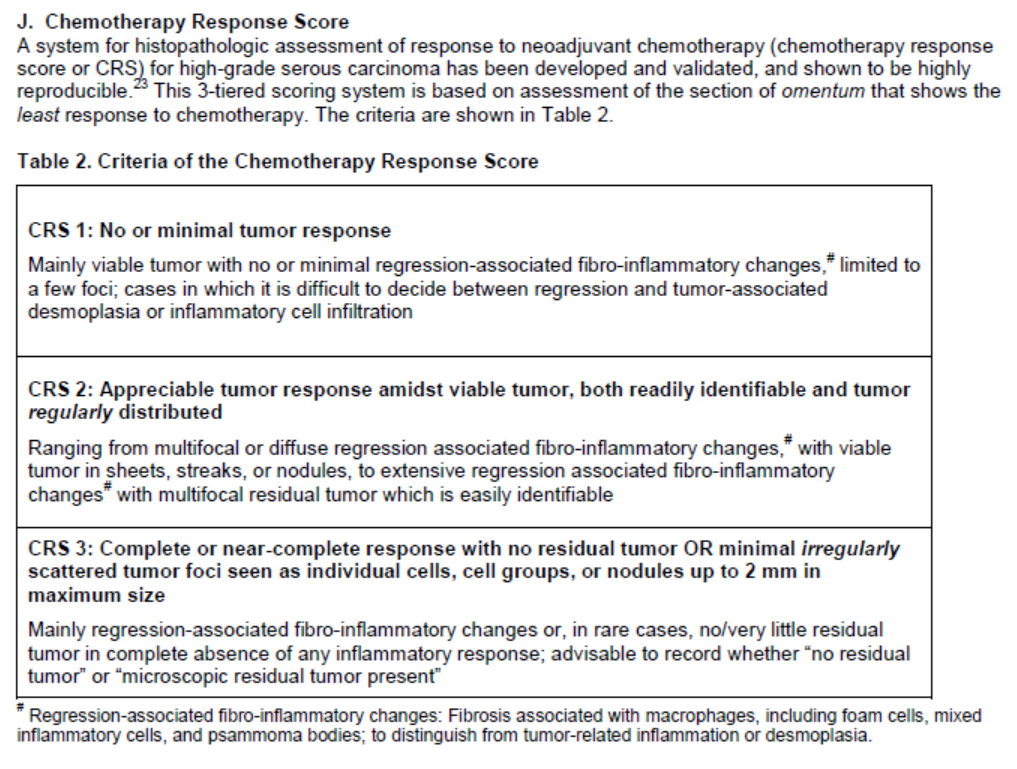
Online Pathology / Laboratory tools
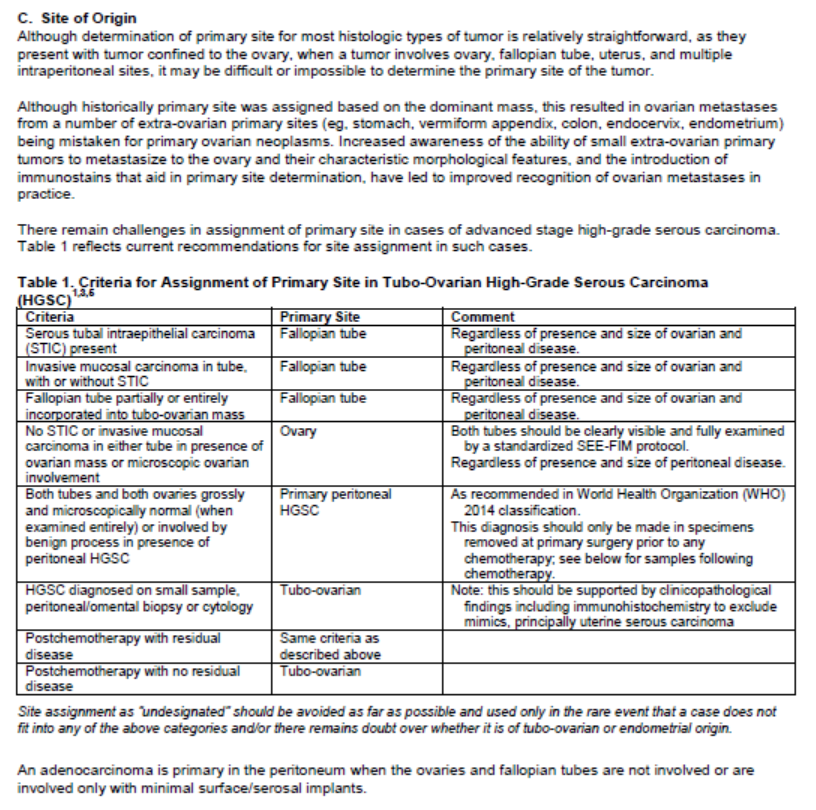
Online Pathology / Laboratory tools

Cap Cancer Templates

Common Alerting Protocol (CAP) Sample Alert Message Templates United

PPT Cancer Pathology and Biomarker Reporting PowerPoint Presentation
Esophagus CAP protocol Esophagus Esophageal Cancer
CAP Cancer Protocol Pancreas Exocrine PDF Páncreas Cáncer

Cancer Protocol Templates College of American Pathologists Sentinel

Cap Cancer Templates
Uterinecervix 4.1.0.0 Protocol Posting Date:
Web Protocol For The Examination Of Specimens From Patients With Primary Carcinoma Of The Uterine Cervix.
June 2021 This Biomarker Template Is Not Required For Accreditation Purposes But May Be Used To Facilitate Compliance With Cap.
Web © 2021 College Of American Pathologists (Cap).
Related Post:

