Clinical Evaluation Plan Template
Clinical Evaluation Plan Template - Web a clinical evaluation plan (cep) is essential for establishing the scope of the clinical evaluation. Web a clinical evaluation plan is a detailed document that outlines the systematic approach and strategy for evaluating the clinical performance and safety of a medical device. The following checklist provides an overview of possible documents and. Web the clinical evaluation plan, or cep, is a crucial starting point and guide for the clinical evaluation of your medical device. Web clinical evaluation assessment report template. Web clinical evaluation assessment report template, specifying recommended minimum content for a notified body. The document is fully editable so that you can adapt it to your company design. Web preview clinical evaluation plan template. Web clinical evaluation plan template. According to the regulation (eu) 2017/745, article 61 and annex xiv, the evaluation of the clinicalperformance and safety as well as the clinical benefit must be based on ‘clinical data’ and is required forall medical device classes. Your complete eu mdr compliance. Web the main task of the clinical evaluation activities is now to strengthen the confidence of these assumptions through the targeted identification of clinical data e.g. Web clinical evaluation assessment report template, specifying recommended minimum content for a notified body. Web a clinical evaluation plan (cep) is essential for establishing the scope of the clinical. Documents include placeholder marks for all. The clinical evaluation report and the clinical data on which it is based,verifies the clinical. The document is fully editable so that you can adapt it to your company design. All medical devices marketed in eu member state countries must undertake. Web preview clinical evaluation plan template. Web preview clinical evaluation plan template. Web clinical evaluation assessment report template. Your complete eu mdr compliance. Learn more about this key document and. It is a critical part of the clinical evaluation documentation as per. According to the regulation (eu) 2017/745, article 61 and annex xiv, the evaluation of the clinicalperformance and safety as well as the clinical benefit must be based on ‘clinical data’ and is required forall medical device classes. Learn more about this key document and. Web clinical evaluation plan according to eu mdr 2017/745. The following checklist provides an overview of possible. Web the clinical evaluation plan, or cep, is a crucial starting point and guide for the clinical evaluation of your medical device. Web clinical evaluation plan according to eu mdr 2017/745. Web templates clinical evaluation templates. Web a clinical evaluation plan is a structured document that outlines how a medical device will be clinically evaluated. Web the primary purpose of. Web a clinical evaluation plan is a structured document that outlines how a medical device will be clinically evaluated. Web the clinical evaluation plan, or cep, is a crucial starting point and guide for the clinical evaluation of your medical device. Your complete eu mdr compliance. The clinical evaluation plan defines methods for creating and updating the clinical evaluation report.. Learn more about this key document and. The clinical evaluation plan defines methods for creating and updating the clinical evaluation report. Documents include placeholder marks for all. Web preview clinical evaluation plan template. Web clinical evaluation assessment report template, specifying recommended minimum content for a notified body. Web clinical evaluation plan template. Web a clinical evaluation plan (cep) is essential for establishing the scope of the clinical evaluation. Web the main task of the clinical evaluation activities is now to strengthen the confidence of these assumptions through the targeted identification of clinical data e.g. All medical devices marketed in eu member state countries must undertake. Web clinical. • conclusions from the clinical evaluation may indicate a. Learn more about this key document and. According to the regulation (eu) 2017/745, article 61 and annex xiv, the evaluation of the clinicalperformance and safety as well as the clinical benefit must be based on ‘clinical data’ and is required forall medical device classes. Web clinical evaluation assessment report template, specifying recommended. Your complete eu mdr compliance. • conclusions from the clinical evaluation may indicate a. All medical devices marketed in eu member state countries must undertake. Web clinical evaluation assessment report template, specifying recommended minimum content for a notified body. Learn more about this key document and. • conclusions from the clinical evaluation may indicate a. The following checklist provides an overview of possible documents and. Web a clinical evaluation plan is a structured document that outlines how a medical device will be clinically evaluated. Web the clinical evaluation plan, or cep, is a crucial starting point and guide for the clinical evaluation of your medical device. It is a critical part of the clinical evaluation documentation as per. Web clinical evaluation plan according to eu mdr 2017/745. Documents include placeholder marks for all. All medical devices marketed in eu member state countries must undertake. Learn more about this key document and. According to the regulation (eu) 2017/745, article 61 and annex xiv, the evaluation of the clinicalperformance and safety as well as the clinical benefit must be based on ‘clinical data’ and is required forall medical device classes. The document is fully editable so that you can adapt it to your company design. Web a clinical evaluation plan is a detailed document that outlines the systematic approach and strategy for evaluating the clinical performance and safety of a medical device. Web a clinical evaluation plan (cep) is essential for establishing the scope of the clinical evaluation. This document has been endorsed by the medical device coordination group (mdcg) established by article 103. Alongside the clinical evaluation report, the clinical evaluation plan. The clinical evaluation report and the clinical data on which it is based,verifies the clinical.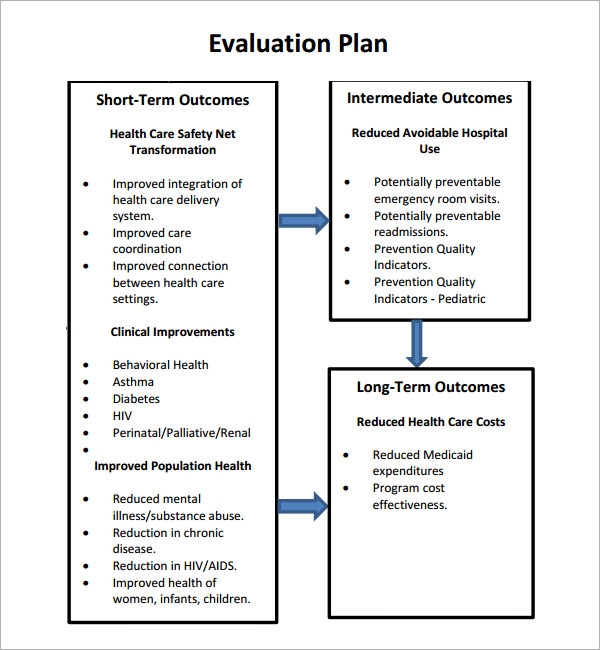
Monitoring And Evaluation Plan Template

Clinical Evaluation Plan Example Form Fill Out and Sign Printable PDF

Free 9 Medical Evaluation Form Samples Templates In Pdf Ms Word
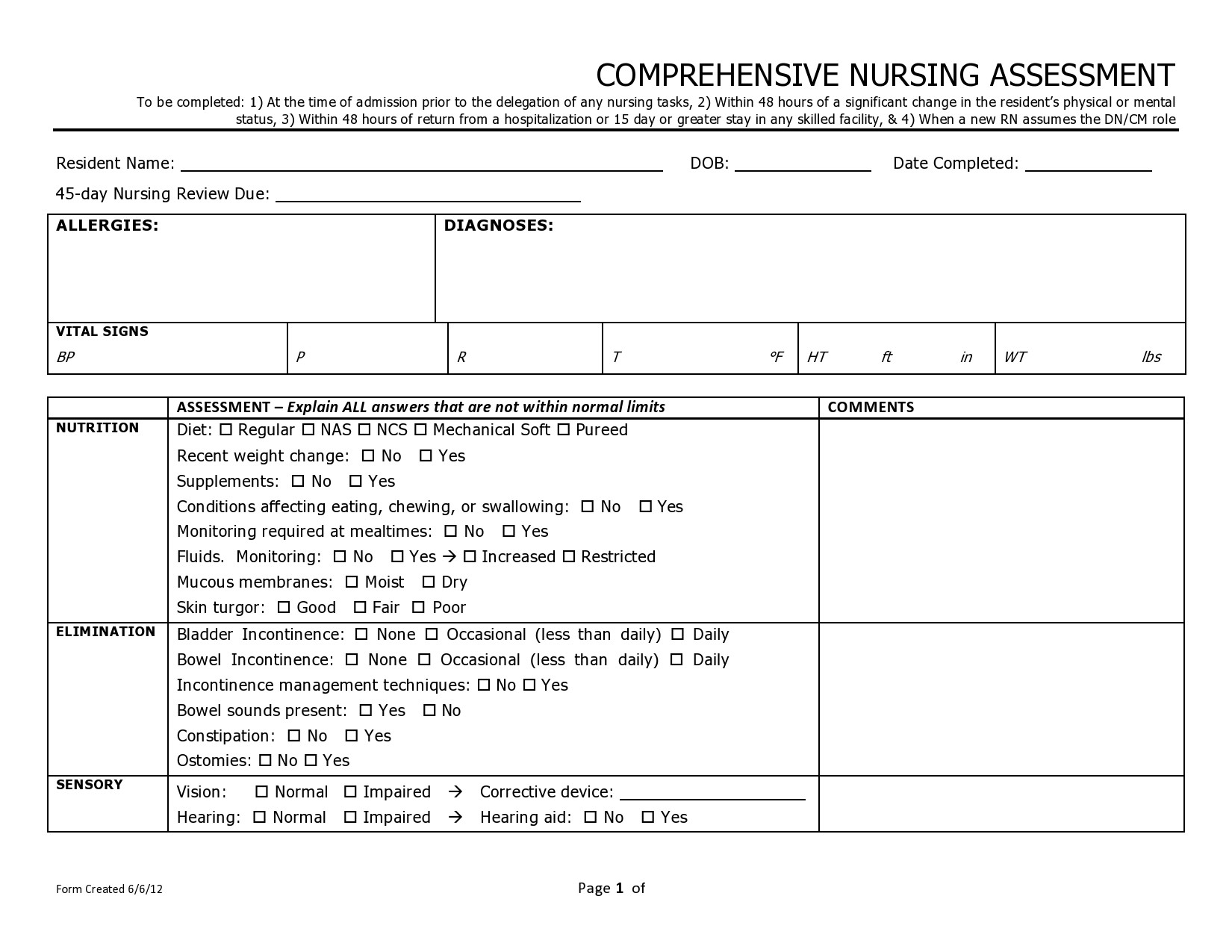
39 Printable Nursing Assessment Forms (+Examples)

Clinical Evaluation Plan Template

Clinical Evaluation Plan (CEP) Template Clinical Study Templates

Clinical Evaluation Plan Template
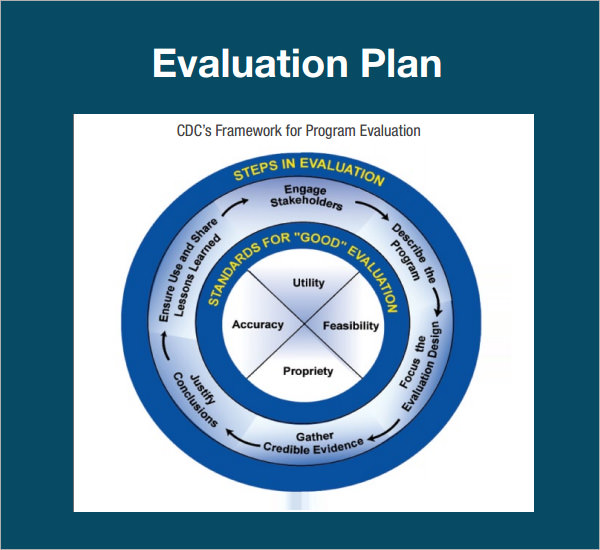
FREE 8+ Evaluation Plan Templates in MS Word PDF

Clinical Evaluation Report Template Mdr

Clinical Evaluation Plan/Report Fill and Sign Printable Template
Your Complete Eu Mdr Compliance.
Web The Primary Purpose Of This Document Is To Provide Manufacturers With Guidance On How To Conduct And Document The Clinical Evaluation Of A Medical Device As Part Of The.
Web Templates Clinical Evaluation Templates.
Web Clinical Evaluation Assessment Report Template, Specifying Recommended Minimum Content For A Notified Body.
Related Post: