Clinical Study Report Template
Clinical Study Report Template - Web the clinical study report described in this guideline is an “integrated” full report of an individual. Web the clinical study report described in this guideline is an integrated full report of an individual study of any therapeutic, prophylactic or diagnostic agent (referred to herein as. Web the clinical study report (csr) is arguably the most important document emerging from a clinical trial. Web what is a clinical study report (csr)? 1 study information study title: Web the niams templates can serve as a starting point for developing study specific reports but the niams does not require they be followed. The template is designed for use in various trial settings. What is a clinical study report? Abbreviated clinical study report development phase: Template version 6.1 jan 20, 2014. Make sure that you are. Web the niams templates can serve as a starting point for developing study specific reports but the niams does not require they be followed. Csrs answer questions such as:. As depicted in the nia guidance on clinical trials, nia is responsible for overseeing the. This guideline can be found. It is not a sales or marketing tool; As depicted in the nia guidance on clinical trials, nia is responsible for overseeing the. Web the niams templates can serve as a starting point for developing study specific reports but the niams does not require they be followed. 1 study information study title: Instead, it is a scientific. Web what is a clinical case report? Web topics included in the report guide cover reporting checklists, trial report structure, choice of title, writing style, trial registry and reporting consistency, spin or reporting. Web the clinical study report described in this guideline is an “integrated” full report of an individual. A clinical study report (csr) is a document that describes. Web the clinical study report (csr) is arguably the most important document emerging from a clinical trial. Web adopt a document template that captures the essential ich e3 requirements and maintain a consistent style* guidelines and statutory requirements change. Web the niams templates can serve as a starting point for developing study specific reports but the niams does not require. Instead, it is a scientific. Ich e3 offers a csr template to guide you in terms of providing the proper data and content in a specified order and format. As depicted in the nia guidance on clinical trials, nia is responsible for overseeing the. Web the niams templates can serve as a starting point for developing study specific reports but. Web clinical study reports (csrs) are often created as part of the process of submitting applications for new medical treatments to regulators. Instead, it is a scientific. Web download a free template for writing a clinical study report from the global health trials' tools and templates library. As depicted in the nia guidance on clinical trials, nia is responsible for. Web the clinical study report described in this guideline is an integrated full report of an individual study of any therapeutic, prophylactic or diagnostic agent (referred to herein as. A clinical study report (csr) is a document that describes the methods and results of a clinical study or trial, along with a. Web clinical study report (csr) is one of. Csrs summarize a study’s data and outcomes to facilitate the evaluation. Web the full clinical study report (csr) encompasses all aspects and details of the research you’ve conducted. Web what is a clinical study report (csr)? This guideline can be found. Web what is a clinical case report? Abbreviated clinical study report development phase: Web topics included in the report guide cover reporting checklists, trial report structure, choice of title, writing style, trial registry and reporting consistency, spin or reporting. Center for drug evaluation and research. Some in the pharmaceutical industry have expressed concern that the ich e3 guidance, structure and content of clinical study reports (ich e3),. Web what is a clinical study report (csr)? Web download a free template for writing a clinical study report from the global health trials' tools and templates library. “integrated full report of an individual study of any therapeutic, prophylactic or diagnostic agent (referred to herein as drug or treatment). The objective of this guideline is to facilitate the compilation of. It is not a sales or marketing tool; A csr is a descriptive account of a. Web topics included in the report guide cover reporting checklists, trial report structure, choice of title, writing style, trial registry and reporting consistency, spin or reporting. Web the niams templates can serve as a starting point for developing study specific reports but the niams does not require they be followed. Csrs summarize a study’s data and outcomes to facilitate the evaluation. The template is designed for use in various trial settings. The objective of this guideline is to facilitate the compilation of a single core clinical study report. This guideline can be found. Web as such, this csr template is the foundation for an “integrated” full report of any study with a therapeutic, prophylactic, or diagnostic agent (i.e., drug or treatment) conducted in. Web clinical study reports (csrs) are often created as part of the process of submitting applications for new medical treatments to regulators. Web what is a clinical case report? Web what is a clinical study report (csr)? Make sure that you are. “integrated full report of an individual study of any therapeutic, prophylactic or diagnostic agent (referred to herein as drug or treatment). Some in the pharmaceutical industry have expressed concern that the ich e3 guidance, structure and content of clinical study reports (ich e3), is intended as a requirement. Instead, it is a scientific.
Editable Clinical Trials Templates For PowerPoint SlideUpLift

Clinical Evaluation Report Template

Clinical Study Report RIAT Support Center
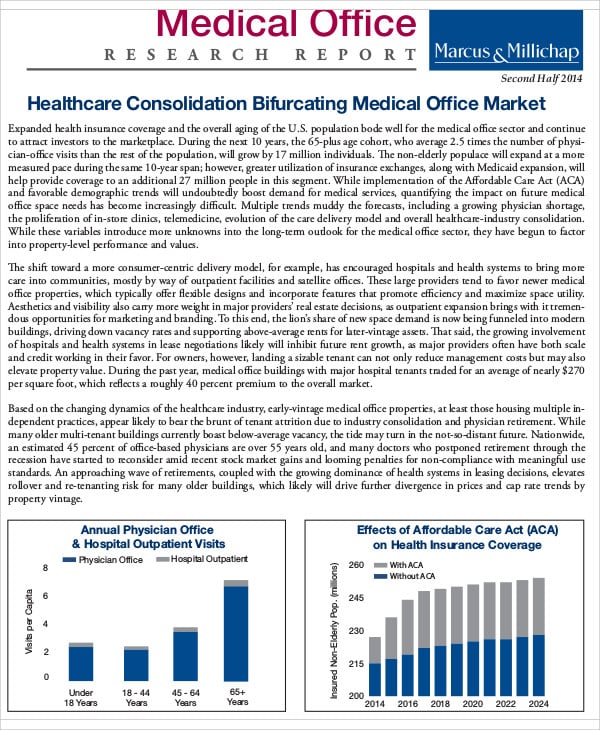
42+ Medical Report Samples Word, PDF, Illustrator

Clinical Trial Report Template (3) TEMPLATES EXAMPLE TEMPLATES

Clinical Study Report (CSR) Template Clinical Study Templates

Clinical Summary Template Fill Online, Printable, Fillable, Blank

Clinical Study Report Template Word

(PDF) Clinical Study Report (CSR) Template DOKUMEN.TIPS
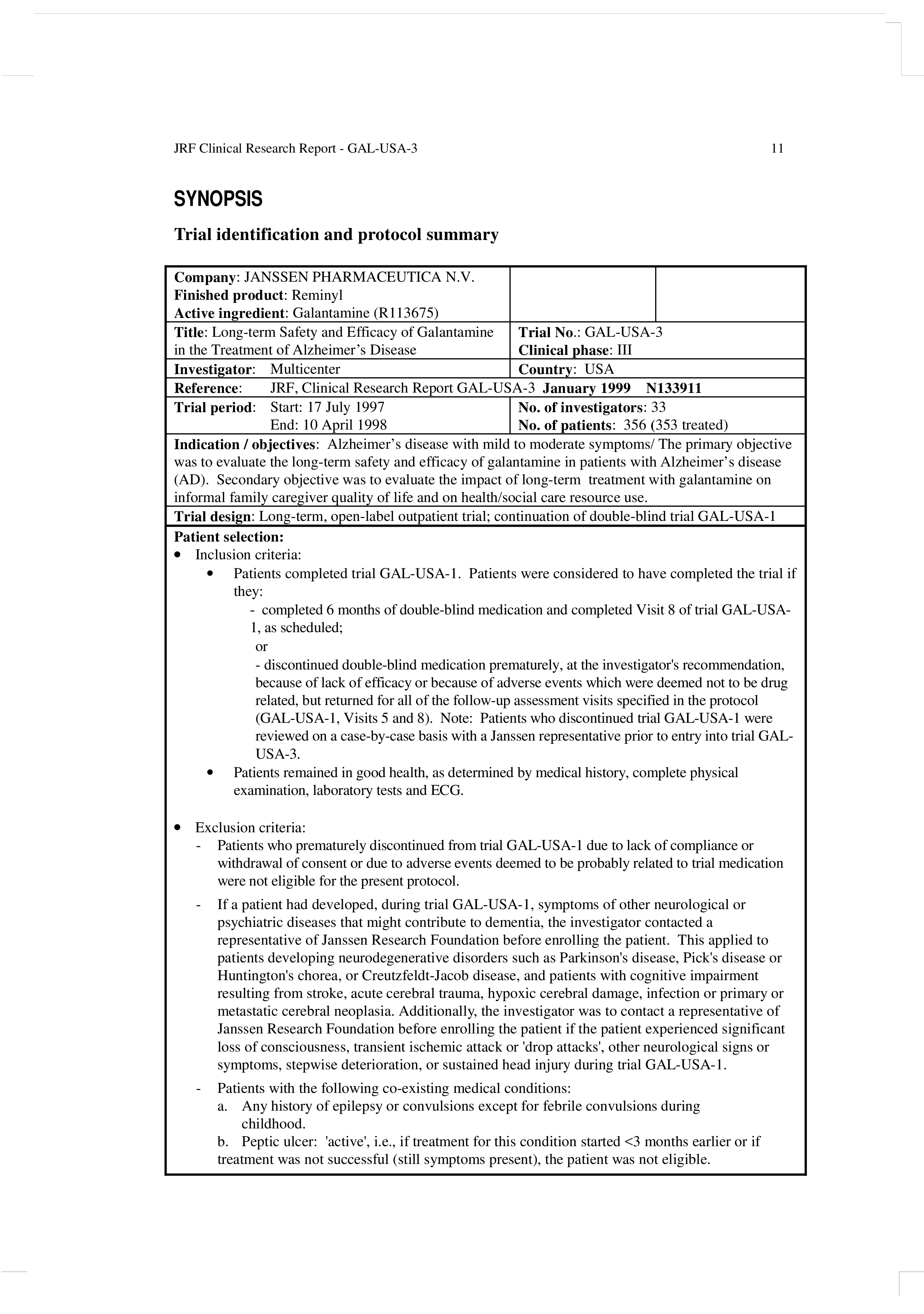
Clinical Research Report Synopsis Templates at
Web The Clinical Study Report Described In This Guideline Is An “Integrated” Full Report Of An Individual.
1 Study Information Study Title:
Csrs Answer Questions Such As:.
Ich E3 Offers A Csr Template To Guide You In Terms Of Providing The Proper Data And Content In A Specified Order And Format.
Related Post: