Clinical Trial Report Template
Clinical Trial Report Template - The purpose, people, and phases of clinical research from 2010 to july 2021, clinicaltrials.gov reports that the number of registered clinical trials. The signatures of the principal or coordinating investigator, the sponsor’s responsible medical officer, and the report. Web clinical study report (csr) is one of many types of regulatory documents that comprise a marketing application for a drug, biologic, or device. A csr is a descriptive account of a. Web center for drug evaluation and research. Web the clinical study report (csr) is arguably the most important document emerging from a clinical trial. Although this guideline is mainly aimed at efficacy and. Case report form, completion guidelines, case report form design, electronic case report form, standard templates. Web anatomy of a clinical trial: The text portion of the sap will be. The objective of this guideline is to facilitate the compilation of a single core clinical study report acceptable to all regulatory authorities. Web the clinical study report (csr) is arguably the most important document emerging from a clinical trial. Csrs summarize a study’s data and outcomes to facilitate the evaluation of. Web anatomy of a clinical trial: Web evaluation (10. Web the sap expands the statistical section of the protocol and contains a detailed description of methods to analyze data collected in the study. The signatures of the principal or coordinating investigator, the sponsor’s responsible medical officer, and the report. Web the clinical study report (csr) is arguably the most important document emerging from a clinical trial. Web anatomy of. Web protocol templates for clinical trials. Although this guideline is mainly aimed at efficacy and. Web anatomy of a clinical trial: Ich e3 offers a csr template to guide you in terms of providing the proper data and content in a specified order and format. Nih applicants can use a template with instructional and sample text to help write clinical. The signatures of the principal or coordinating investigator, the sponsor’s responsible medical officer, and the report. Please note that this page has been updated for 2015 following a quality check and review of the templates, and. Forms, tools, & templates description category (ies) keyword (s) 02.04.02 investigator's brochure. Web the toolbox contains templates, sample forms, guidelines, regulations and informational materials. Web protocol templates for clinical trials. Web print these checklists, templates, and examples to help gather information needed to report results to clinicaltrials.gov. This template aims to facilitate the development of phase. Web center for drug evaluation and research. Learn how to prepare tables, listings, and figures for clinical study report and submit to regulatory agencies, the. If none were used, this should be stated. Ich e3 offers a csr template to guide you in terms of providing the proper data and content in a specified order and format. Web as such, this csr template is the foundation for an “integrated” full report of any study with a therapeutic, prophylactic, or diagnostic agent (i.e., drug or treatment). The purpose, people, and phases of clinical research from 2010 to july 2021, clinicaltrials.gov reports that the number of registered clinical trials. Web the integrated full report of a study should not be derived by simply joining a separate clinical and statistical report. Web anatomy of a clinical trial: The text portion of the sap will be. Case report form,. Web center for drug evaluation and research. Ich e3 offers a csr template to guide you in terms of providing the proper data and content in a specified order and format. Web the clinical study report (csr) is arguably the most important document emerging from a clinical trial. A csr is a descriptive account of a. Web protocol templates for. If none were used, this should be stated. Web evaluation (10 minutes) what is a clinical study report (csr)? Web the clinical study report (csr) is arguably the most important document emerging from a clinical trial. This guideline can be found. This template aims to facilitate the development of phase. Cloud based technologycapture and managefuture of life scienceagile development Web topics included in the report guide cover reporting checklists, trial report structure, choice of title, writing style, trial registry and reporting consistency, spin or reporting. Please note that this page has been updated for 2015 following a quality check and review of the templates, and. If none were used, this. Web evaluation (10 minutes) what is a clinical study report (csr)? Although this guideline is mainly aimed at efficacy and. Forms, tools, & templates description category (ies) keyword (s) 02.04.02 investigator's brochure. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of. Case report form, completion guidelines, case report form design, electronic case report form, standard templates. This document aims to allow the compilation of a single core clinical study report acceptable to all. Web novartis staff analyzed this study and authored this report. Web the integrated full report of a study should not be derived by simply joining a separate clinical and statistical report. Ich e3 offers a csr template to guide you in terms of providing the proper data and content in a specified order and format. Web the sap expands the statistical section of the protocol and contains a detailed description of methods to analyze data collected in the study. Cloud based technologycapture and managefuture of life scienceagile development This template aims to facilitate the development of phase. Please note that this page has been updated for 2015 following a quality check and review of the templates, and. If none were used, this should be stated. A csr is a descriptive account of a. “integrated full report of an individual study of any therapeutic, prophylactic or diagnostic agent (referred to herein as.
Clinical Trial Report Template TEMPLATES EXAMPLE TEMPLATES EXAMPLE
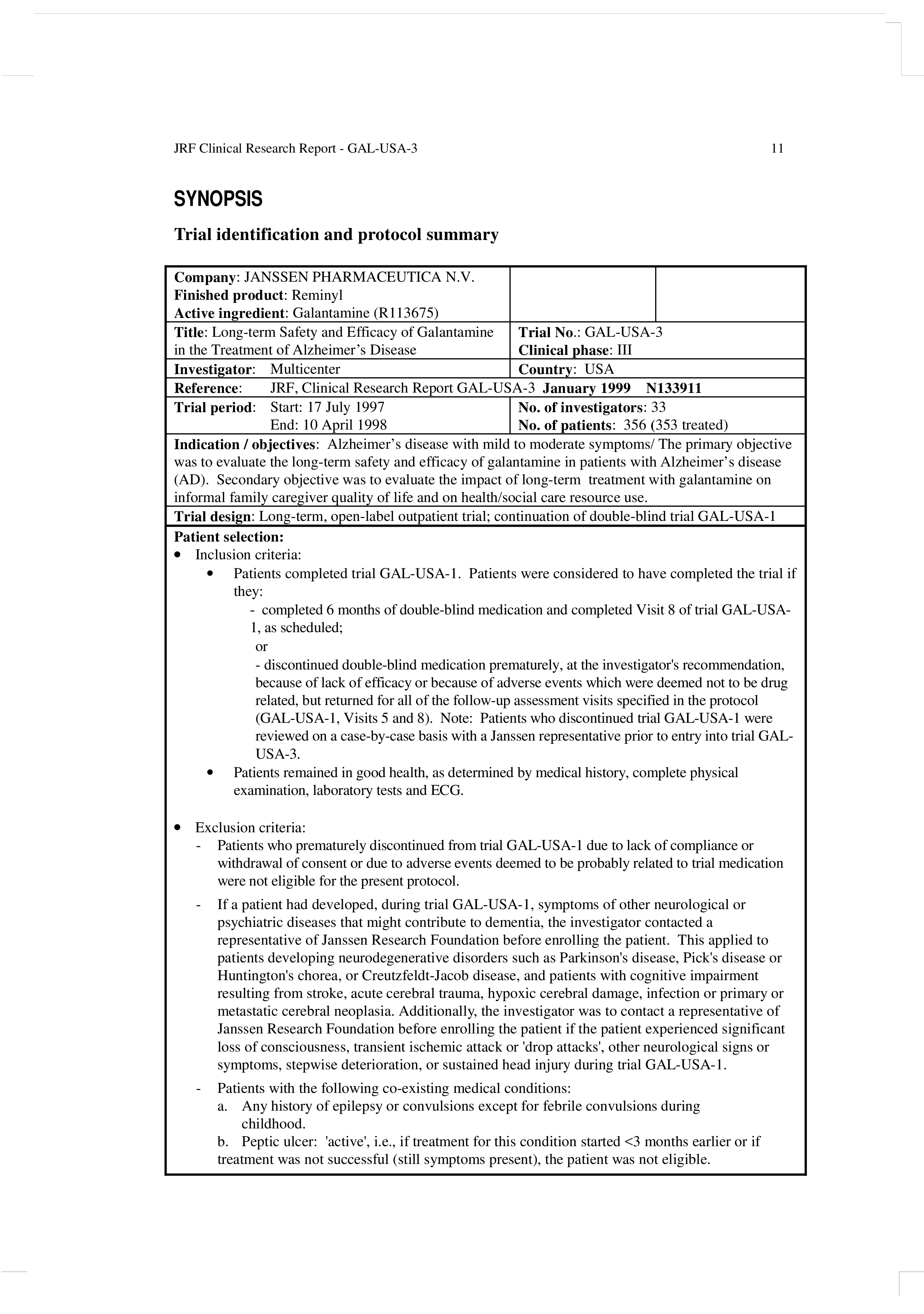
Clinical Research Report Synopsis Templates at

Clinical Trial Report Template (3) PROFESSIONAL TEMPLATES

Clinical Trial Report Template
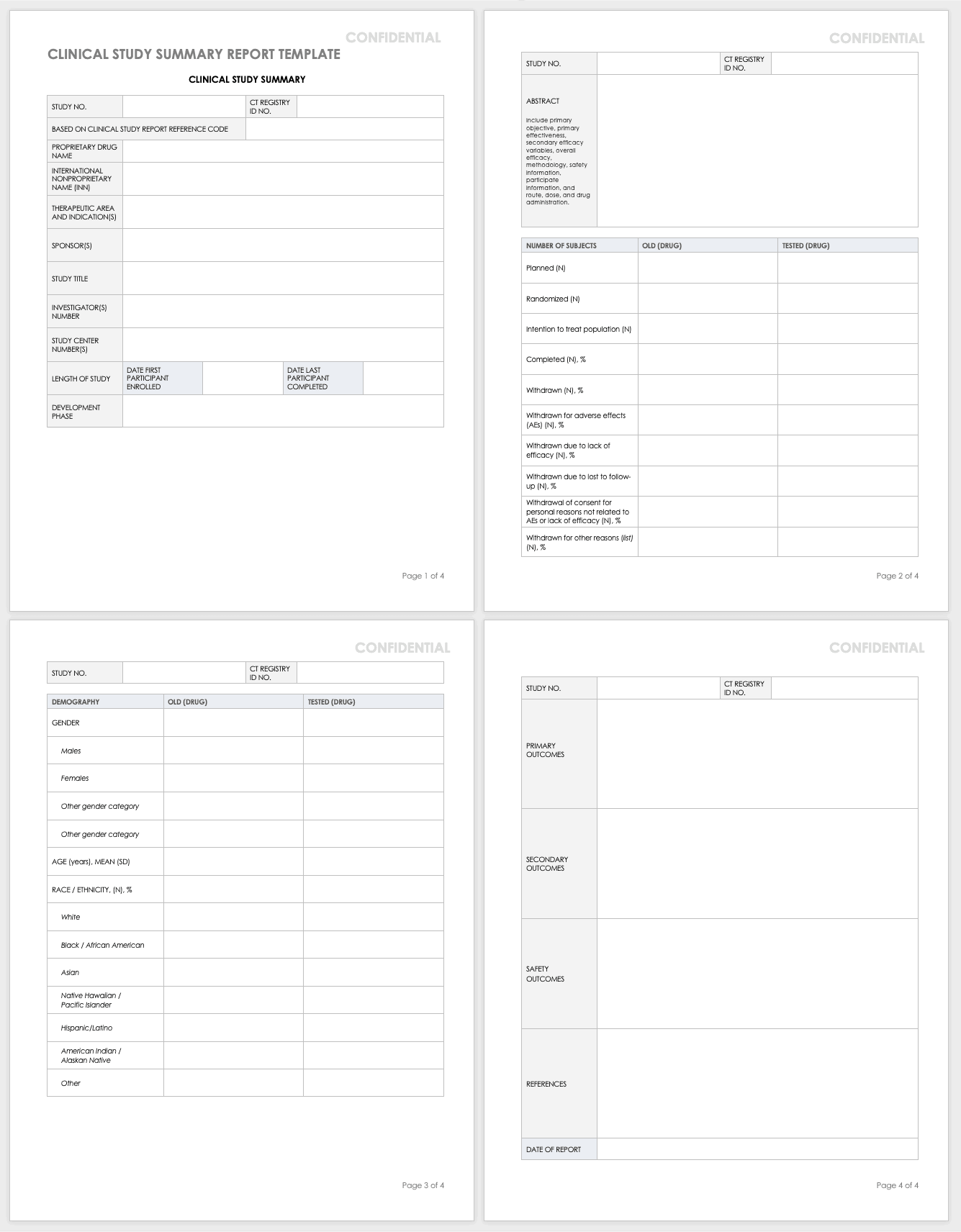
Free Clinical Trial Templates Smartsheet

Case Report Form Template Clinical Trials
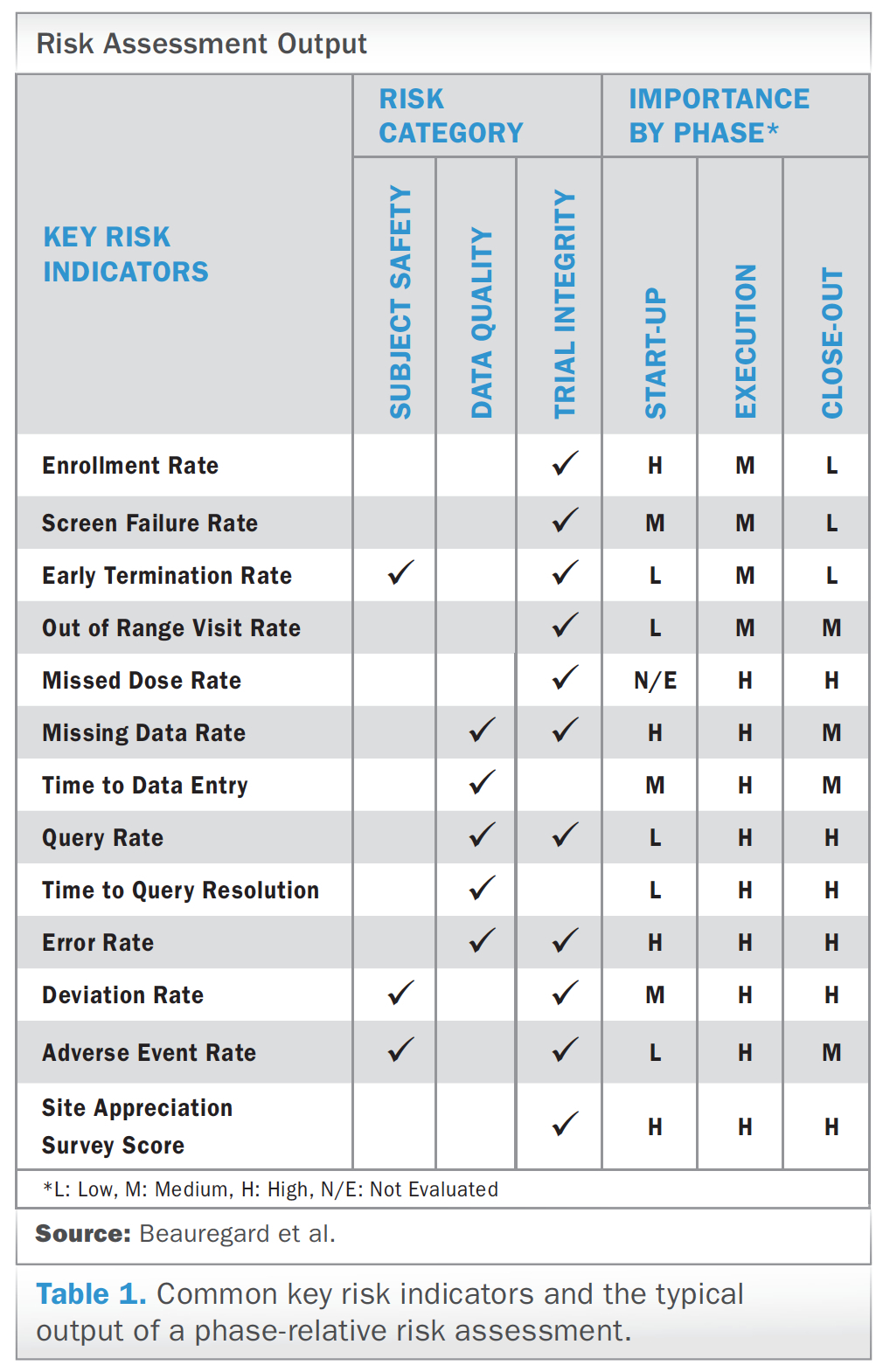
Monitoring Report Template Clinical Trials
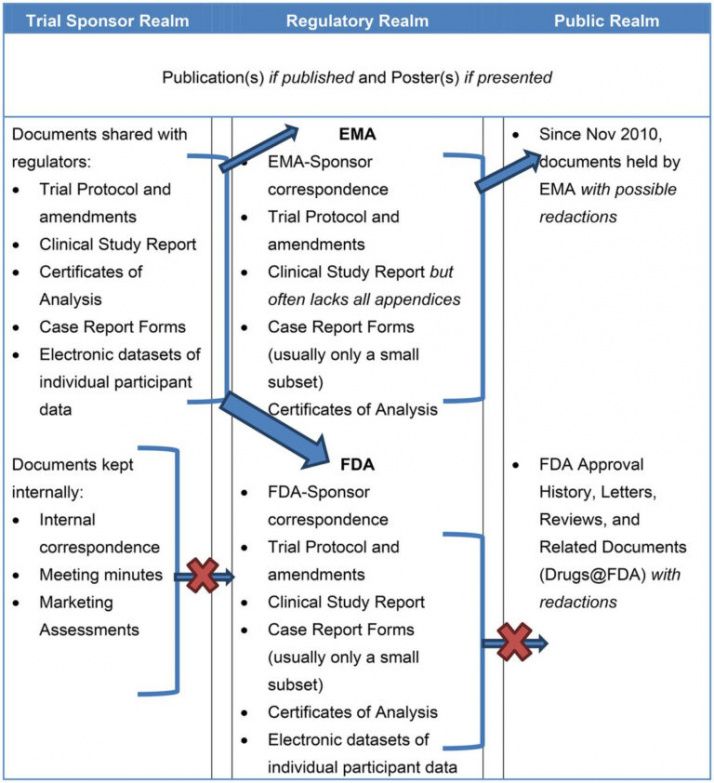
Clinical Study Report Template PDF Sample Stableshvf
Case Eport Form Electronic Qolty Format Ppt In Clinical With Trial

Clinical Trial Report Template 3 Templates Example Templates
Web Print These Checklists, Templates, And Examples To Help Gather Information Needed To Report Results To Clinicaltrials.gov.
Web The Toolbox Contains Templates, Sample Forms, Guidelines, Regulations And Informational Materials To Assist Investigators In The Development And Conduct Of High Quality Clinical.
Web Anatomy Of A Clinical Trial:
Web Protocol Templates For Clinical Trials.
Related Post: