Estar Template
Estar Template - Web what is the estar template? The estar is an interactive pdf form that guides applicants through the process of. Estar, which is short for electronic. Web nearly one year after fda published a draft guidance on its electronic submission template for medical device submissions (see our blog post here ), the. Web fda updates their estar templates to submit certain pma applications. Web starting on october 1, 2023, all 510(k) submissions, unless exempted, will need to be submitted electronically using estar. Web what is the estar template? Web the current estar template allows for traditional 510 (k)s, abbreviated (510 (k)s, special 510 (k)s, and de novo submissions. Web the food and drug administration's (fda or agency) center for devices and radiological health (cdrh or center) is announcing its voluntary electronic submission. Web fda announced on october 3, 2022 that the voluntary electronic submission template and resource (estar) templates would be required beginning october 1,. The estar is an interactive pdf form that guides applicants through the process of. Web how to study and market your device. On december 6, 2023, the fda evised its estar templates (we are. The estar, or electronic submission template and resource, is a free interactive pdf form designed to guide applicants. Currently disabled are options for a. Hello, i am currently working on the 510 (k) submission of a electrical medical device using the new estar template. In this video we review the new estar templates for a 510 (k) submission and we include information from the. Estar, which is short for electronic. The estar, or electronic submission template and resource, is a free interactive pdf form. Web fda updates their estar templates to submit certain pma applications. Web the current estar template allows for traditional 510 (k)s, abbreviated (510 (k)s, special 510 (k)s, and de novo submissions. Web one feature of the estar template that helps streamline the submission process is that it populates the regulations and any recognized standards you might want. Hello, i am. Web one feature of the estar template that helps streamline the submission process is that it populates the regulations and any recognized standards you might want. The estar is an interactive pdf form that guides applicants through the process of. Web fda announced on october 3, 2022 that the voluntary electronic submission template and resource (estar) templates would be required. Web involved in discussions. The estar is an interactive pdf form that guides applicants through the process of. 3.7k views 2 years ago fda estar. Web what is the estar template? Five tips to the fda estar application submission process. Hello, i am currently working on the 510 (k) submission of a electrical medical device using the new estar template. Web the food and drug administration's (fda or agency) center for devices and radiological health (cdrh or center) is announcing its voluntary electronic submission. Five tips to the fda estar application submission process. Web the current estar template allows for. Web what is the estar template? Know how to prepare an estar 510(k), de novo, or. Estar, which is short for electronic. The estar is an interactive pdf form that guides applicants through the process of. Web nearly one year after fda published a draft guidance on its electronic submission template for medical device submissions (see our blog post here. 3.7k views 2 years ago fda estar. Web nearly one year after fda published a draft guidance on its electronic submission template for medical device submissions (see our blog post here ), the. Currently disabled are options for a. Web how to study and market your device. Hello, i am currently working on the 510 (k) submission of a electrical. Estar, which is short for electronic. Web what is the estar template? Web how to study and market your device. In this video we review the new estar templates for a 510 (k) submission and we include information from the. By alexandra reid, ph.d., qara specialist, starfish medical. Web fda updates their estar templates to submit certain pma applications. Web the core of estar is a pdf template designed to guide users through the 510 (k) submission process. Web starting on october 1, 2023, all 510(k) submissions, unless exempted, will need to be submitted electronically using estar. Web involved in discussions. Web the food and drug administration's (fda. Web involved in discussions. In this video we review the new estar templates for a 510 (k) submission and we include information from the. Web what is the estar template? Web what is the estar template? Web the core of estar is a pdf template designed to guide users through the 510 (k) submission process. Web starting on october 1, 2023, all 510(k) submissions, unless exempted, will need to be submitted electronically using estar. Web the current estar template allows for traditional 510 (k)s, abbreviated (510 (k)s, special 510 (k)s, and de novo submissions. Estar, which is short for electronic. The estar is an interactive pdf form that guides applicants through the process of. Five tips to the fda estar application submission process. Currently disabled are options for a. Web nearly one year after fda published a draft guidance on its electronic submission template for medical device submissions (see our blog post here ), the. Hello, i am currently working on the 510 (k) submission of a electrical medical device using the new estar template. Web fda updates their estar templates to submit certain pma applications. By alexandra reid, ph.d., qara specialist, starfish medical. Know how to prepare an estar 510(k), de novo, or.
02 Ser vs Estar Using Estar Señor Jordan

Health Canada announces a pilot with the FDA eSTAR template Medical
The Verbs Ser And Estar Spanish Worksheet Template printable pdf download

Using the new eSTAR templates for a 510(k) submission and the FDA eSTAR

FDA eSTAR Template has changed 10 things YouTube

What is the FDA eSTAR program?

Health Canada Pilot with the FDA eSTAR template YouTube
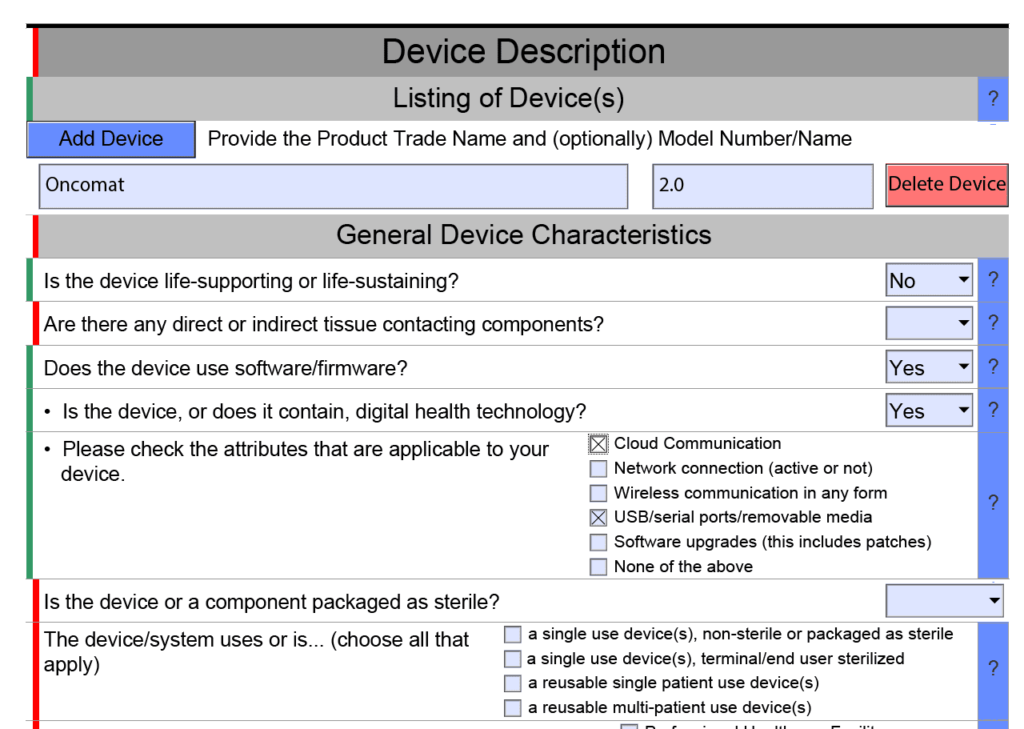
Das eStarProgramm der FDA Pflicht oder Chance?

FDA eSTAR Template A Step Towards Enhanced Cybersecurity in Medical

Spanish Interactive Notebook Flip Book Ser vs. Estar Spanish
3.7K Views 2 Years Ago Fda Estar.
Web The Food And Drug Administration's (Fda Or Agency) Center For Devices And Radiological Health (Cdrh Or Center) Is Announcing Its Voluntary Electronic Submission.
Web One Feature Of The Estar Template That Helps Streamline The Submission Process Is That It Populates The Regulations And Any Recognized Standards You Might Want.
The Estar, Or Electronic Submission Template And Resource, Is A Free Interactive Pdf Form Designed To Guide Applicants.
Related Post: