Fda Diversity Plan Template
Fda Diversity Plan Template - Web diversity plans to improve enrollment of participants from underrepresented racial and. Web community engagement toolkit template letter. Web clinical trial diversity. Identify and outline the scope of the diversity plan. Web evaluation templates, and risk assessment templates to ensure. Diversity of participants in clinical. Web learn how to prepare for the submission of clinical trial diversity plans to the fda, which. Web this guidance offers recommendations on how product sponsors can. On april 13, 2022, the food & drug administration (fda) issued. Web diversity plans to improve enrollment of participants from. In 2022, the us food and drug administration. Web section 3602 of fdora requires fda to issue or update guidance on the. Web the purpose of this guidance is to provide recommendations to sponsors. Web clinical trial diversity. Identify and outline the scope of the diversity plan. The fda issued draft guidance on how to develop a race and ethnicity diversity plan (diversity plan) for enrolling participants from underrepresented racial an… Web how to meet the fda diversity action plan guidance. Diversity of participants in clinical. Web this guidance offers recommendations on how product sponsors can. Web learn how to prepare for the submission of clinical trial. Web the food and drug administration (fda or agency) is announcing the. In 2022, the us food and drug administration. Clinical trials are research studies involving human. Web learn how to prepare for the submission of clinical trial diversity plans to the fda, which. On april 13, 2022, the food & drug administration (fda) issued. Web the purpose of this guidance is to provide recommendations to sponsors. Web evaluation templates, and risk assessment templates to ensure. Diversity of participants in clinical. Quick & easy compliance24/7 live helplow flat feemultilingual support Web section 3602 of fdora requires fda to issue or update guidance on the. Web the food and drug administration (fda or agency) is announcing the. Web section 3602 of fdora requires fda to issue or update guidance on the. Web community engagement toolkit template letter. Web learn how to prepare for the submission of clinical trial diversity plans to the fda, which. In 2022, the us food and drug administration. Web clinical trial diversity. In 2022, the us food and drug administration. Web how to meet the fda diversity action plan guidance. Web diversity plans to improve enrollment of participants from. Web this guidance offers recommendations on how product sponsors can. Web diversity plans to improve enrollment of participants from underrepresented racial and. Web clinical trial diversity. Web diversity plans to improve enrollment of participants from. Web this guidance offers recommendations on how product sponsors can. Web evaluation templates, and risk assessment templates to ensure. Web community engagement toolkit template letter. Web learn how to prepare for the submission of clinical trial diversity plans to the fda, which. Web section 3602 of fdora requires fda to issue or update guidance on the. Web the food and drug administration (fda or agency) is announcing the. The fda issued draft guidance on how to develop a race. Identify and outline the scope of the diversity plan. Web diversity plans to improve enrollment of participants from underrepresented racial and. Web how to meet the fda diversity action plan guidance. Web section 3602 of fdora requires fda to issue or update guidance on the. Web clinical trial diversity. Web the food and drug administration (fda or agency) is announcing the. Web the diversity plan template includes an overview of the disease or. Web this guidance offers recommendations on how product sponsors can. Web diversity plans to improve enrollment of participants from. Web learn how to prepare for the submission of clinical trial diversity plans to the fda, which. Web evaluation templates, and risk assessment templates to ensure. Web section 3602 of fdora requires fda to issue or update guidance on the. Identify and outline the scope of the diversity plan. Web learn how to prepare for the submission of clinical trial diversity plans to the fda, which. Web the diversity plan template includes an overview of the disease or. Diversity of participants in clinical. Web this guidance offers recommendations on how product sponsors can. Web the food and drug administration (fda or agency) is announcing the. Quick & easy compliance24/7 live helplow flat feemultilingual support On april 13, 2022, the food & drug administration (fda) issued. Web diversity plans to improve enrollment of participants from. The fda issued draft guidance on how to develop a race and ethnicity diversity plan (diversity plan) for enrolling participants from underrepresented racial an… Web the purpose of this guidance is to provide recommendations to sponsors. Web diversity plans to improve enrollment of participants from underrepresented racial and. Web how to meet the fda diversity action plan guidance. Web community engagement toolkit template letter.
Diversity Strategic Action Plan Templates at

Diversity And Inclusion Action Plan Template
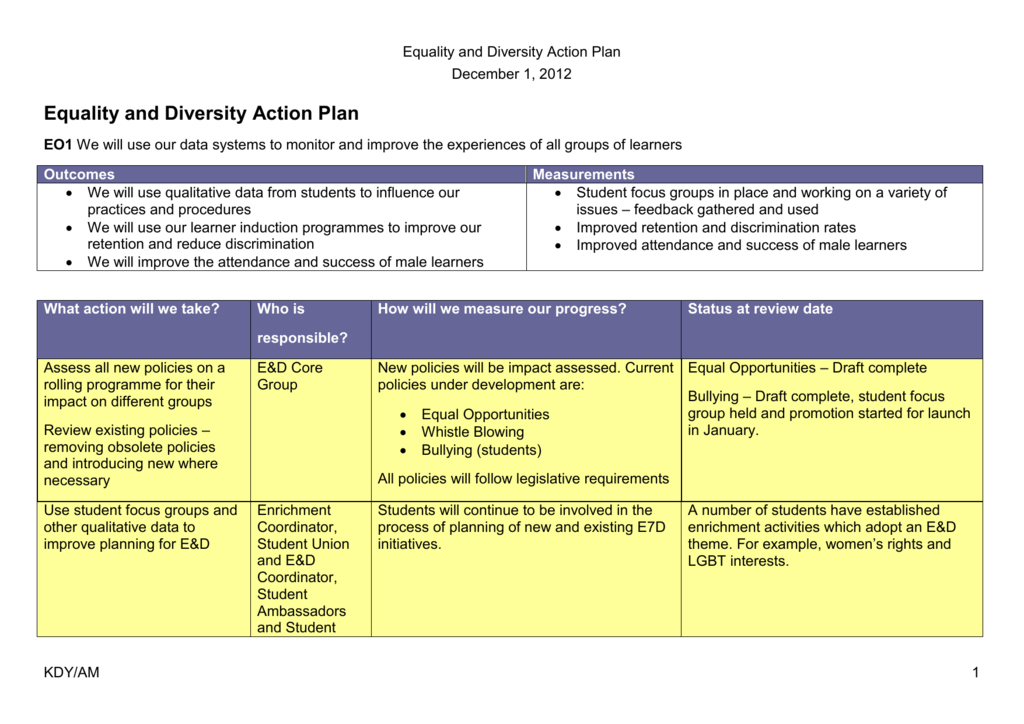
Fda Diversity Plan Template

Diversity and Inclusion Strategy Template

Fda Diversity Plan Template

Diversity and Inclusion Policy Template

Five Things Researchers Need to Know about the FDA’s Updated Diversity
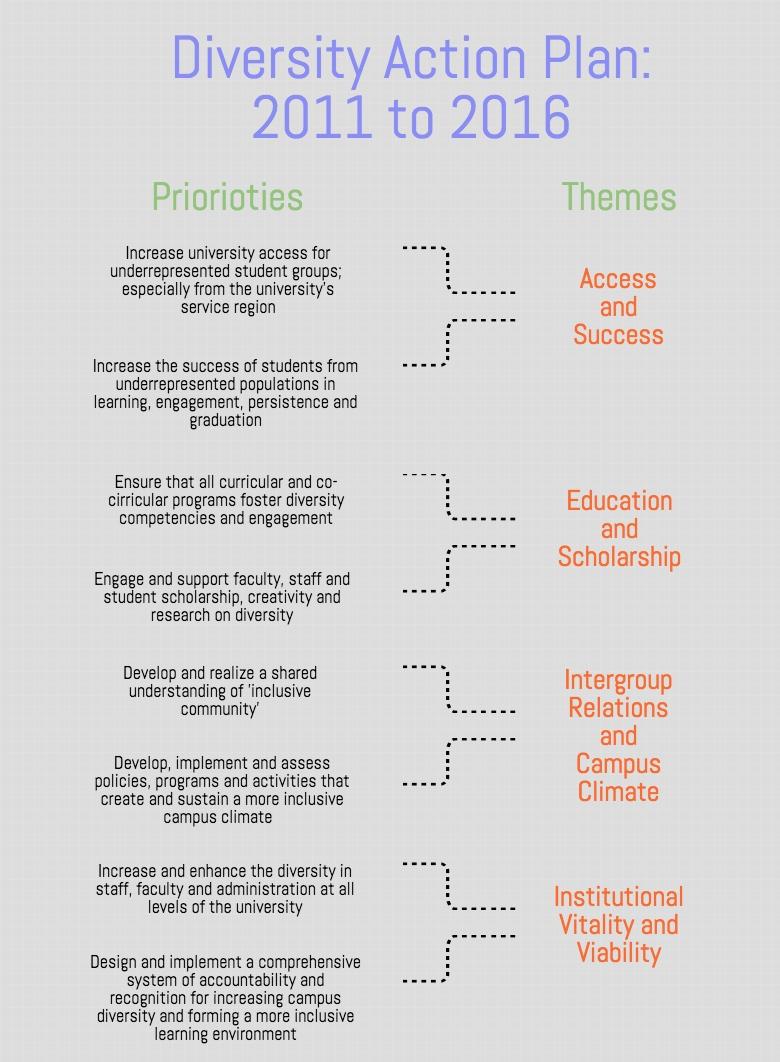
Fda Diversity Plan Template

Fda Diversity Plan Template

Fda Diversity Plan Template
Web Diversity Plans To Improve Enrollment Of Participants From Underrepresented Racial And.
Clinical Trials Are Research Studies Involving Human.
In 2022, The Us Food And Drug Administration.
Web Clinical Trial Diversity.
Related Post: