Gspr Checklist Template
Gspr Checklist Template - Web designed to support your conformity to annex i of mdr 2017/745, this document contains a full table of mdr general safety and performance requirements as well as a list of. Mention the international standard (preferably harmonized) or the common specification (cs) applicable. The general safety and performance requirements that apply to the device and an. (just in case if no one shares any template here) actually it is not difficult to create your own checklist. This excel spreadsheet is designed to support manufacturers making the transition from mdd to mdr / ivdd to ivdr. Web 4easyreg has made available a gspr checklist that will help you to ensure compliance of your devices and related documentation with the safety and performance requirements. Web medical device regulation 2017/745 general essential safety and performance requirements check list template. Page 1 of 10 # requirement standards applied design documentation qualification Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Web we offer an intuitive gspr checklist template, customised to accommodate your unique ivd medical devices. This template acts as an invaluable tool in easing the. The completed checklist must include: Page 1 of 10 # requirement standards applied design documentation qualification When a requirement applies, a simple statement can. Web we offer an intuitive gspr checklist template, customised to accommodate your unique ivd medical devices. The completed checklist must include: Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. This template acts as an invaluable tool in easing the. Web medical device regulation 2017/745 general essential safety and performance requirements check list template. This excel spreadsheet is designed to support. Mention the international standard (preferably harmonized) or the common specification (cs) applicable. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. It is planned that these templates will be withdrawn once the eudamed module for clinical. “reduce as far as possible and appropriate the risks. “reduce as far as possible and appropriate the risks from unintended cuts and pricks, such as needle stick injuries”. Web we offer an intuitive gspr checklist template, customised to accommodate your unique ivd medical devices. Web designed to support your conformity to annex i of mdr 2017/745, this document contains a full table of mdr general safety and performance requirements. Web we offer an intuitive gspr checklist template, customised to accommodate your unique ivd medical devices. It is planned that these templates will be withdrawn once the eudamed module for clinical. This excel spreadsheet is designed to support manufacturers making the transition from mdd to mdr / ivdd to ivdr. Web mdrg has created a general safety & performance requirements. Web mdrg has created a general safety & performance requirements checklist that contains a full table of the requirements, along with a list of applicable standards. This excel spreadsheet is designed to support manufacturers making the transition from mdd to mdr / ivdd to ivdr. (just in case if no one shares any template here) actually it is not difficult. Web every single section of the eu mdr or ivdr gspr should be assessed in its own right, as it pertains to your medical device. It is planned that these templates will be withdrawn once the eudamed module for clinical. Web mdrg has created a general safety & performance requirements checklist that contains a full table of the requirements, along. This excel spreadsheet is designed to support manufacturers making the transition from mdd to mdr / ivdd to ivdr. Web some topics such as clinical evaluation and medicinal consultation have moved from the requirements list into the articles, while other topics are new to the requirements list,. (just in case if no one shares any template here) actually it is. Web what should you include in your gspr checklist? “reduce as far as possible and appropriate the risks from unintended cuts and pricks, such as needle stick injuries”. Mention the international standard (preferably harmonized) or the common specification (cs) applicable. Web in the gspr checklist clearly mention the below points: This template acts as an invaluable tool in easing the. Web what should you include in your gspr checklist? Page 1 of 10 # requirement standards applied design documentation qualification Web medical device regulation 2017/745 general essential safety and performance requirements check list template. Web in the gspr checklist clearly mention the below points: Web some topics such as clinical evaluation and medicinal consultation have moved from the requirements list. This template acts as an invaluable tool in easing the. Web we offer an intuitive gspr checklist template, customised to accommodate your unique ivd medical devices. When a requirement applies, a simple statement can. “reduce as far as possible and appropriate the risks from unintended cuts and pricks, such as needle stick injuries”. (just in case if no one shares any template here) actually it is not difficult to create your own checklist. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Web some topics such as clinical evaluation and medicinal consultation have moved from the requirements list into the articles, while other topics are new to the requirements list,. This excel spreadsheet is designed to support manufacturers making the transition from mdd to mdr / ivdd to ivdr. The general safety and performance requirements that apply to the device and an. Web designed to support your conformity to annex i of mdr 2017/745, this document contains a full table of mdr general safety and performance requirements as well as a list of. Web what should you include in your gspr checklist? Page 1 of 10 # requirement standards applied design documentation qualification Web planned to be conducted as to any specific national requirements. Web medical device regulation 2017/745 general essential safety and performance requirements check list template. It is planned that these templates will be withdrawn once the eudamed module for clinical. Mention the international standard (preferably harmonized) or the common specification (cs) applicable.
MDR 2017/745 GSPR template Easy Medical Device School
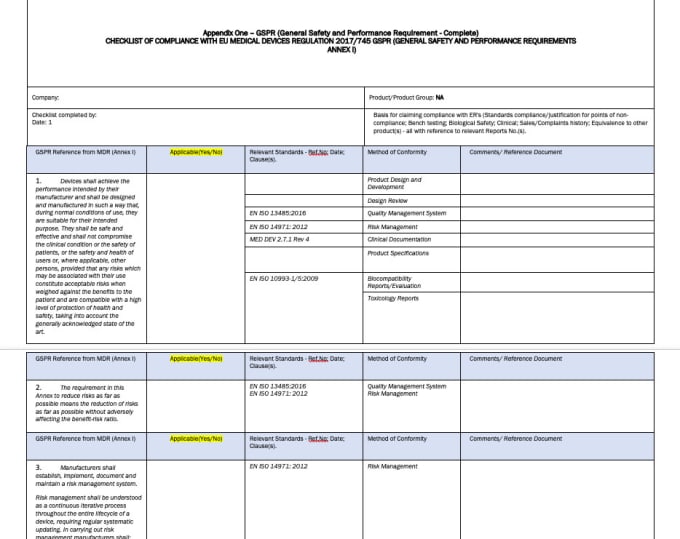
Mdr Gspr Checklist Template Portal Tutorials
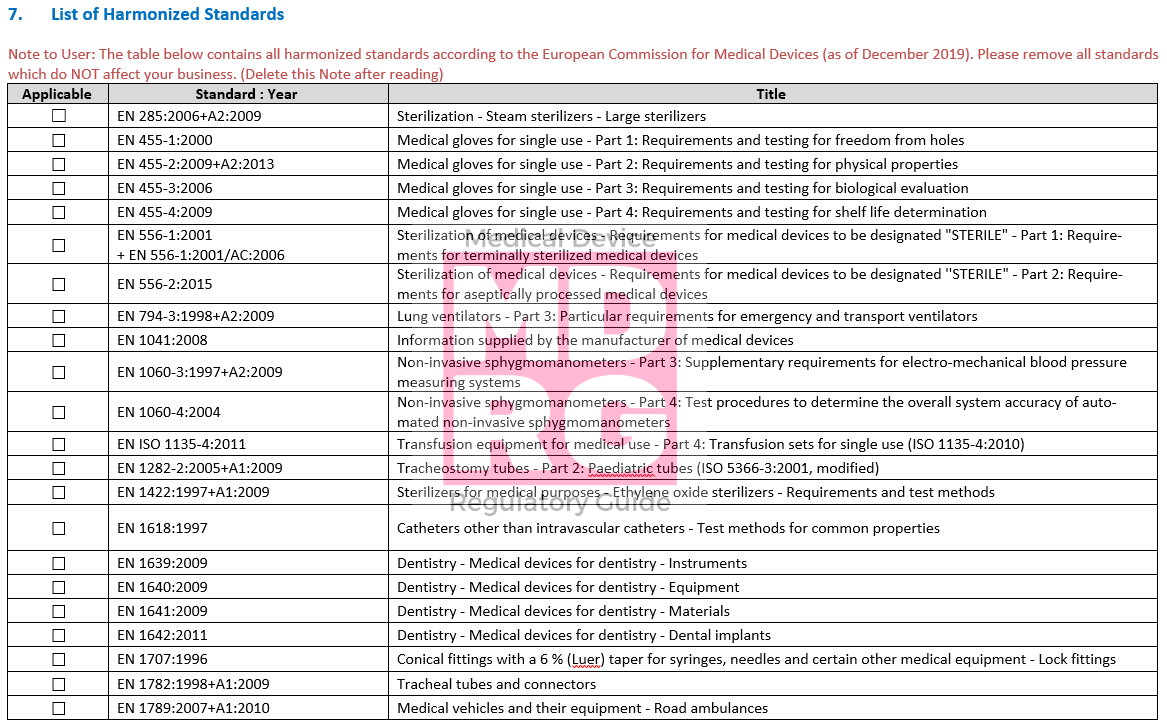+GSPR+Checklist+-+SCREENSHOT+2+WATERMARK.png)
General Safety and Performance Requirements (GSPR) Checklist — Medical

Comparative table GSPR Essential Requirements (v3) Académie DM Experts
![Free IVDR GSPR Checklist [Template] Trinzo](https://static.wixstatic.com/media/51879b_59a5cf39e38c4181a0b12fde3ab7cfb3~mv2.png/v1/fill/w_2240,h_1260,al_c/51879b_59a5cf39e38c4181a0b12fde3ab7cfb3~mv2.png)
Free IVDR GSPR Checklist [Template] Trinzo

Mdr Gspr Checklist Template Printable Word Searches
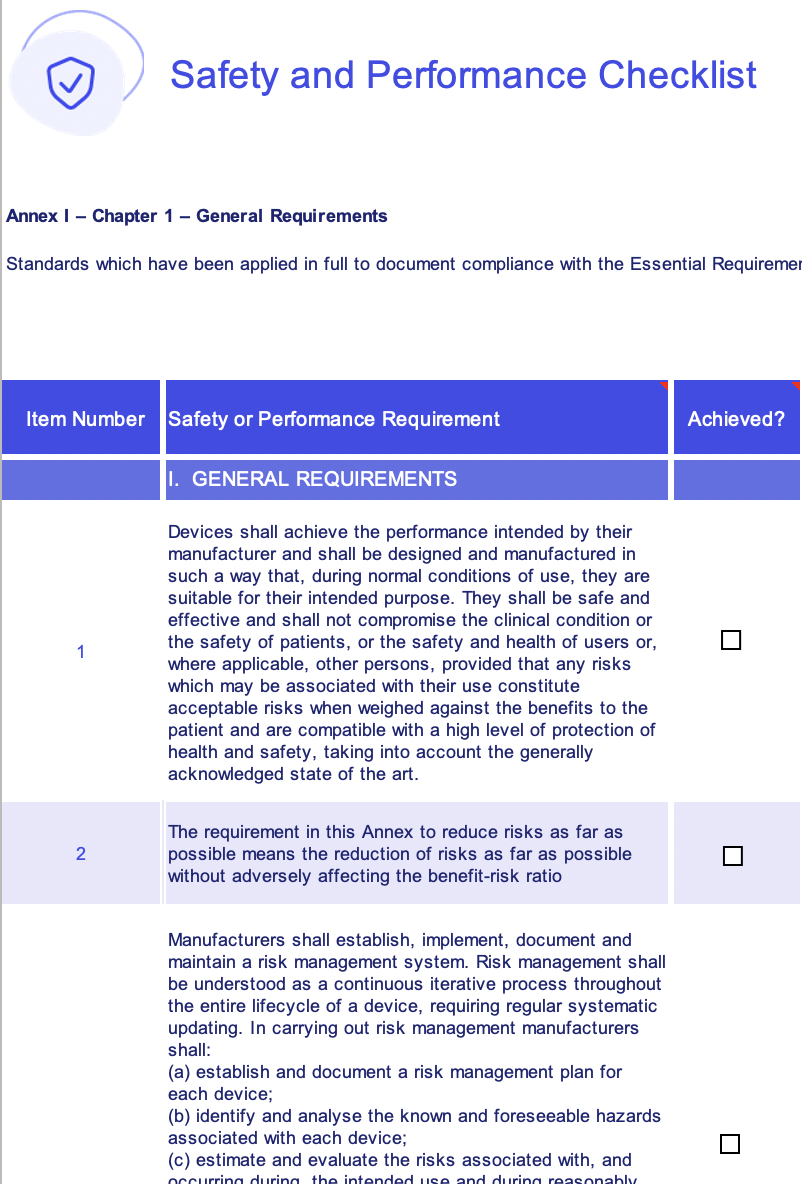
EU MDR general safety and performance requirements (GSPR) checklist
+GSPR+Checklist+-+SCREENSHOT+1+WATERMARK.png)
General Safety and Performance Requirements (GSPR) Checklist — Medical
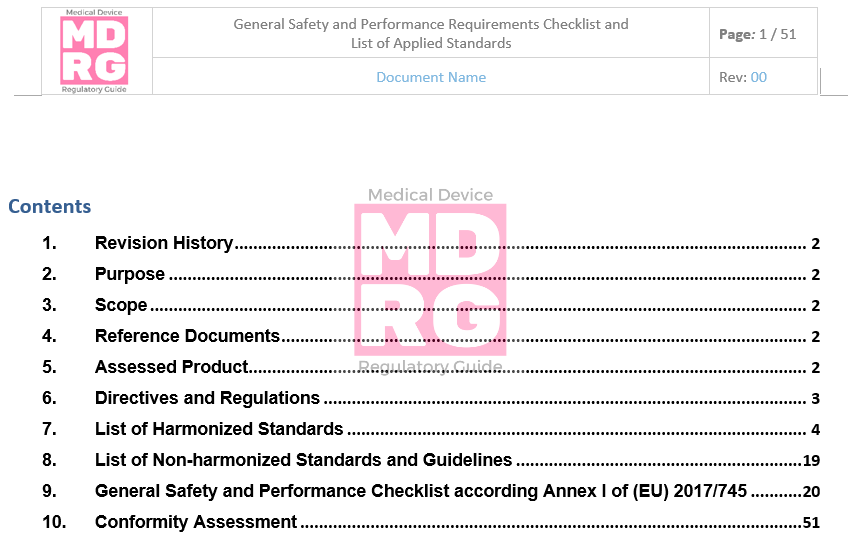
MDR General Safety and Performance (GSPR) checklist
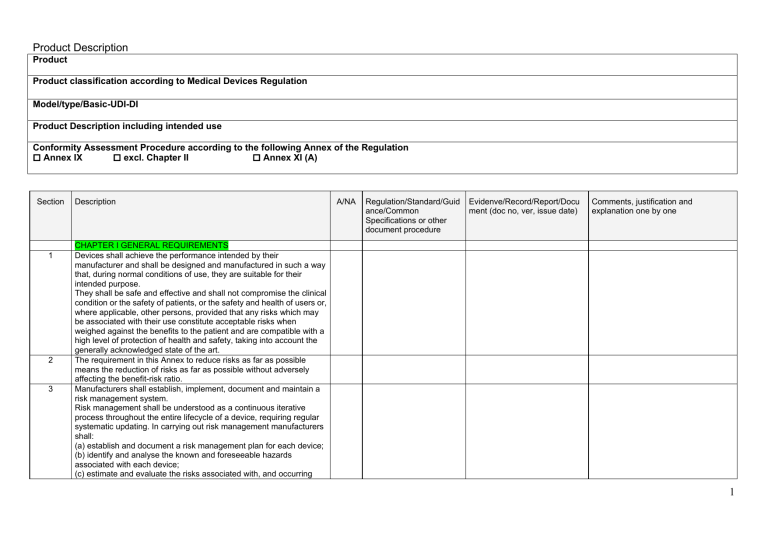
GSPR checklist
Web In The Gspr Checklist Clearly Mention The Below Points:
Web Every Single Section Of The Eu Mdr Or Ivdr Gspr Should Be Assessed In Its Own Right, As It Pertains To Your Medical Device.
Web 4Easyreg Has Made Available A Gspr Checklist That Will Help You To Ensure Compliance Of Your Devices And Related Documentation With The Safety And Performance Requirements.
Web Trusted Information Resource.
Related Post: