Hipaa Form Template
Hipaa Form Template - 1.1 hipaa statement for international research form: Entity or business associate, you don’t have to comply with the hipaa rules. Web your health care provider and health plan must give you a notice that tells you how they may use and share your health information. You can also get a copy from the carepatron app or our resources. All clinical trial protocols being submitted to wcg irb are required to use the following templates: The federal health insurance portability and accountability act of 1996 (hipaa) and state laws mandate that health providers not disclose a patient’s information without valid. Authorization for use and disclosure of health information for research : A notice with the design elements found in the booklet, but formatted for full page presentation. It must also include your health privacy rights. The reader should consult with knowledgeable legal counsel to determine how applicable laws apply to the reader’s specific circumstances. The details usually consist of what phi is being shared, why it is being shared, who it is being shared. Notify of a security breach (60 days) make agreements with subcontractors. The document, also known as a “health insurance portability and accountability act (hipaa)” form, must satisfy the. Web sharing medical records (3 rules) when sharing medical records, three rules. The federal health insurance portability and accountability act of 1996 (hipaa) and state laws mandate that health providers not disclose a patient’s information without valid. A patient can also request their medical records not currently in their possession. Adult authorization to contact you about future. Use our ready to go form templates or build your own hipaa compliant online forms. Adult authorization to contact you about future. These agencies are responsible for protecting my rights. Web the medical record information release (hipaa) form allows patients to give authorization to a 3rd party and access their health records. Web sample business associate agreement provisions. Wcg irb informed consent form. Web so far the forms plus great customer support feel like a good fit for me. Entity or business associate, you don’t have to comply with the hipaa rules. Web effective december 23, 2024, a group health plan may want to consider taking the following actions to comply with the reproductive health care rules: These agencies are responsible for protecting. To allow the authorized party to communicate with me for marketing purposes when they receive payment from a third party. All clinical trial protocols being submitted to wcg irb are required to use the following templates: Entity or business associate, you don’t have to comply with the hipaa rules. Web sharing medical records (3 rules) when sharing medical records, three. The following terms used in this agreement shall have the same meaning as those terms in the hipaa rules: You can also ask for a copy at any time. Entity or business associate, you don’t have to comply with the hipaa rules. Notify of a security breach (60 days) make agreements with subcontractors. Follow these steps to get started: A notice with the design elements found in the booklet, but formatted for full page presentation. Entity or business associate, you don’t have to comply with the hipaa rules. Follow these steps to get started: Web a medical records release authorization form is a document that allows a person to disclose protected health information to a third party. Web so. A patient can also request their medical records not currently in their possession. This resource is provided for informational and reference purposes only and should not be construed as the legal advice of the american medical. To allow the authorized party to communicate with me for marketing purposes when they receive payment from a third party. Web wcg irb (formerly. Powers granted under a medical release can be revoked or reassigned at any time. A layered notice that presents a summary of the information on the first page, followed by the full content on the following pages; Notify of a security breach (60 days) make agreements with subcontractors. For the acquisition of an adult’s written consent to participate in research.. Entity or business associate, you don’t have to comply with the hipaa rules. Web notice in the form of a booklet (preferred by consumers in focus testing); The reader should consult with knowledgeable legal counsel to determine how applicable laws apply to the reader’s specific circumstances. Follow these steps to get started: A text only version of the notice. Use our ready to go form templates or build your own hipaa compliant online forms with our easy drag and drop form designer. The document, also known as a “health insurance portability and accountability act (hipaa)” form, must satisfy the. Covered entities and business associates must follow hipaa rules. Web hipaa and part 2; Web the medical record information release (hipaa) form allows patients to give authorization to a 3rd party and access their health records. All clinical trial protocols being submitted to wcg irb are required to use the following templates: This is a sample form document intended solely for general informational purposes. It does not constitute legal advice. For the acquisition of an adult’s written consent to participate in research. Post fillable interactive web or pdf forms online with esignature, file upload, and more. Notify of a security breach (60 days) make agreements with subcontractors. Wcg irb informed consent form. Learn your rights under hipaa, how your information may be used or shared, and how to file a complaint if you think your rights were violated. Web sample form of hipaa notice of privacy practices disclaimer: Web sample business associate agreement provisions. The federal health insurance portability and accountability act of 1996 (hipaa) and state laws mandate that health providers not disclose a patient’s information without valid.
FREE 8+ Sample Hipaa Release Forms in PDF MS Word
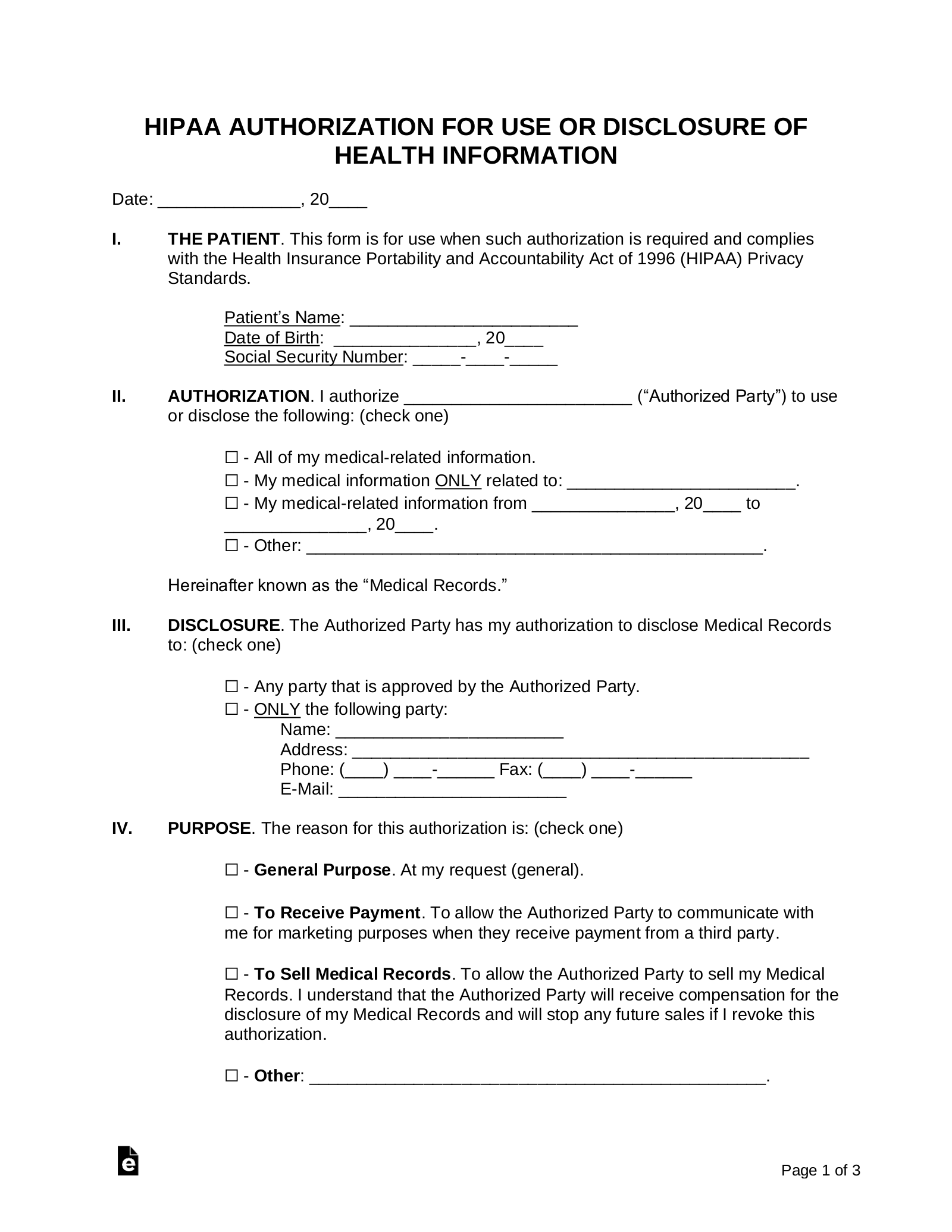
Free Printable Hipaa Forms Printable Templates

FREE 9+ Sample Hipaa Forms in PDF MS Word

Hipaa Policy Templates Free

Printable Hipaa Release Form

FREE 11+ Sample HIPAA Forms in PDF MS Word

FREE 9+ Sample Hipaa Forms in PDF MS Word

Printable HIPAA Consent Form Template Digital Download Etsy Canada

FREE 8+ Sample Hipaa Release Forms in PDF MS Word

Free Printable Hipaa Consent Forms Templates Printable
Web Notice In The Form Of A Booklet (Preferred By Consumers In Focus Testing);
Web Forms And Templates Hipaa Authorization Template.
Notify Of A Security Breach (60 Days) If Patient Information Is Breached, It’s Required To Be Reported Within 60 Days By The.
It Must Also Include Your Health Privacy Rights.
Related Post: