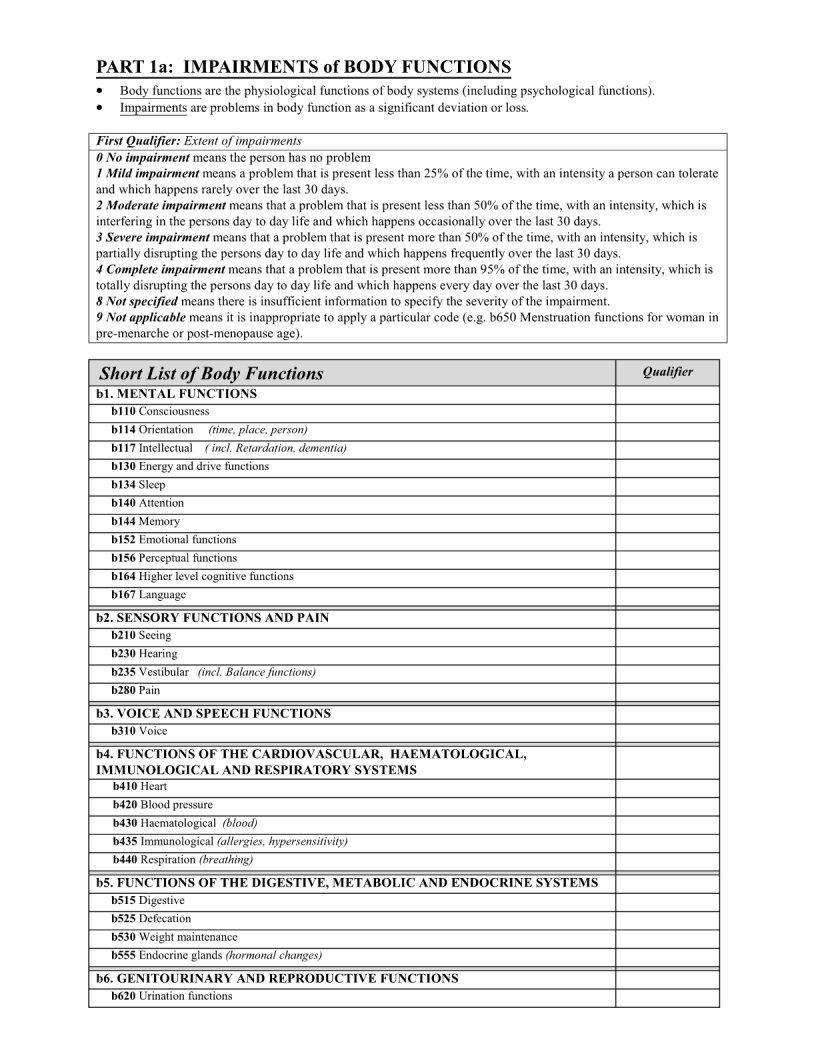
Icf Template
Icf Template - The icf template contains all of the required elements of informed consent per the revised common rule. This template was developed by a dedicated working. Web these new and improved icf templates replace all previous versions posted to our site as of may 17th, 2023; Web you need to use an informed consent form template for research. Web whenever you are proposing research with human participants you must provide a form, known as an informed consent form (icf), with each proposal to indicate that the. Cohort intervention treatment 1 gma301 300 mg (n=9) drug: Web the templates on this page are intended to help investigators construct documents that are as short as possible and written in plain language. Written by editor on 30 september 2020. Web consent templates for use at nih sites. These documents are periodically updated by the irb, so be sure to always use the currently. This information is provided to. Web the icf checklist is a practical tool to elicit and record information on the functioning and disability of an individual. Web the center for health literacy at the university of arkansas for medical sciences (uams) previously developed a plain language informed consent form (pl. Cohort intervention treatment 1 gma301 300 mg (n=9) drug: Written. Specific requirements vary by credential level. This information is provided to. Web how the protocol (plan of research) works, what risks or discomforts they may experience, participation being a voluntary decision on their part. As you review the information below, please ensure that you click on the correct tab for your credentialing level. Cohort intervention treatment 1 gma301 300 mg. This information can be summarized for case. Consent for your child to take part in a research study. Advarra’s experts have the ability to. Icf was officially endorsed by all 191 who member states in the fifty. Web the icf checklist is a practical tool to elicit and record information on the functioning and disability of an individual. Web icf template interventional trials adult patients. Web icf is the who framework for measuring health and disability at both individual and population levels. The icf template contains all of the required elements of informed consent per the revised common rule. The informed consent form (icf) template for clinical trials has been updated to reflect the change in the retention. Web whenever you are proposing research with human participants you must provide a form, known as an informed consent form (icf), with each proposal to indicate that the. Web please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed consent forms (icf). Web the irb provides template consent. This information is provided to. Web the icf checklist is a practical tool to elicit and record information on the functioning and disability of an individual. As you review the information below, please ensure that you click on the correct tab for your credentialing level. Web the templates on this page are intended to help investigators construct documents that are. Applicants for the icf acc, pcc and mcc credentials must meet designated coaching experience requirements for eligibility. Cohort intervention treatment 1 gma301 300 mg (n=9) drug: Web guidance for informed consent documentation and process, short forms in other languages, guidance on the who can serve as interpreters, and our stamping. This informed consent form template is based on fda guidance. Often the use of a specific template is obligatory. Consent for your child to take part in a research study. As of may 17th, 2023, the icf templates available on our informed consent templates page replaces all previously released templates. Web you need to use an informed consent form template for research. Specific requirements vary by credential level. Web these new and improved icf templates replace all previous versions posted to our site as of may 17th, 2023; Web icf template interventional trials adult patients. Advarra’s experts have the ability to. This informed consent form template is based on fda guidance investigational device exemptions (ides) for early feasibility medical device clinical. Web icf is the who framework for. Web update april 20, 2022: Specific requirements vary by credential level. What template you need to use, is. Web icf is the who framework for measuring health and disability at both individual and population levels. Web consent templates for use at nih sites. Icf was officially endorsed by all 191 who member states in the fifty. Web the icf checklist is a practical tool to elicit and record information on the functioning and disability of an individual. What template you need to use, is. Web the center for health literacy at the university of arkansas for medical sciences (uams) previously developed a plain language informed consent form (pl. Web please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed consent forms (icf). Web you need to use an informed consent form template for research. Consent for your child to take part in a research study. Web icf is the who framework for measuring health and disability at both individual and population levels. As of may 17th, 2023, the icf templates available on our informed consent templates page replaces all previously released templates. These documents are periodically updated by the irb, so be sure to always use the currently. Web guidance for informed consent documentation and process, short forms in other languages, guidance on the who can serve as interpreters, and our stamping. As you review the information below, please ensure that you click on the correct tab for your credentialing level. Web consent templates for use at nih sites. Specific requirements vary by credential level. Cohort intervention treatment 1 gma301 300 mg (n=9) drug: Advarra’s experts have the ability to.
Icf Model Template Fill Online, Printable, Fillable, Blank pdfFiller

PPT Application of the ICF in the assessment of the RESPIRATORY
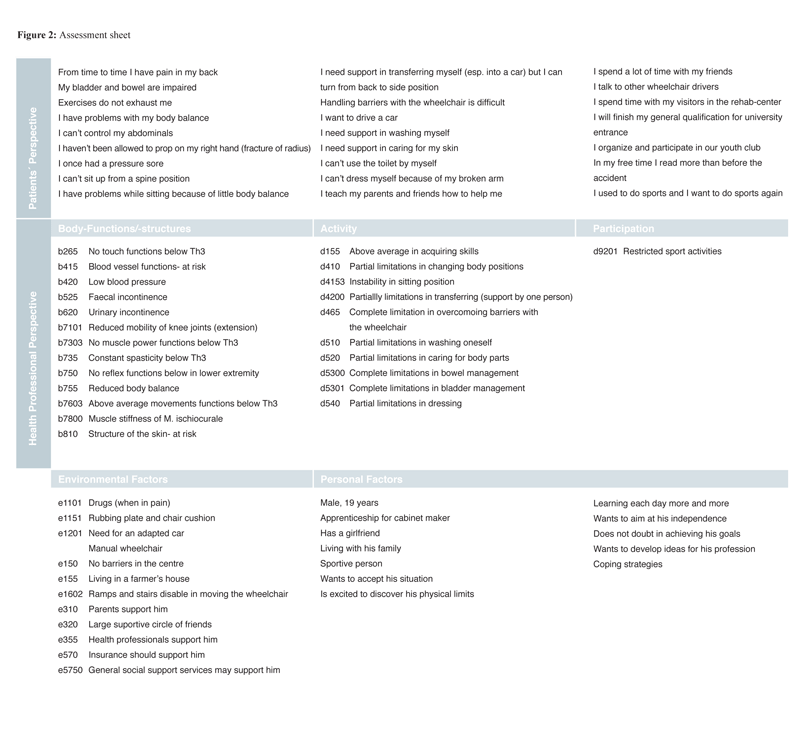
ICF Case Studies The ICF Assessment Sheet
Icf Checklist ≡ Fill Out Printable PDF Forms Online
.png)
International Classification of Functioning, Disability and Health (ICF

Blank ICF Chart

Building an ICF model template YouTube
Blank Icf Checklist Fill Out and Print PDFs

icf model example , Disability and Health (ICF) Framework and
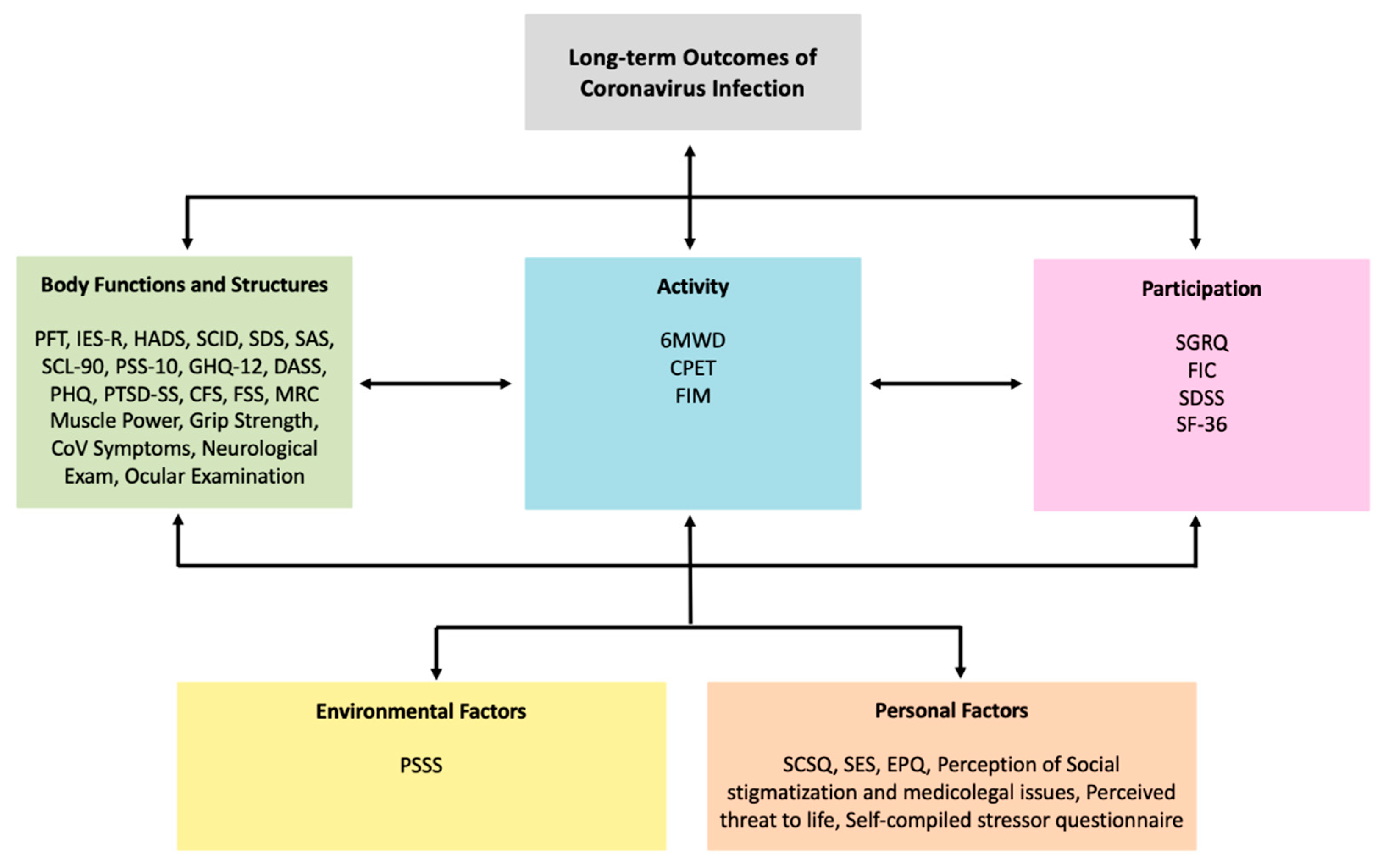
IJERPH Free FullText Applying the WHO ICF Framework to the
Web Update April 20, 2022:
The Icf Template Contains All Of The Required Elements Of Informed Consent Per The Revised Common Rule.
Web How The Protocol (Plan Of Research) Works, What Risks Or Discomforts They May Experience, Participation Being A Voluntary Decision On Their Part.
Web The Templates On This Page Are Intended To Help Investigators Construct Documents That Are As Short As Possible And Written In Plain Language.
Related Post: