Iqcp Template
Iqcp Template - The type and frequency of the quality control may not be less stringent. Web assess the likelihood or probability of harm for each failure and the severity of harm to a patient for each failure. The asm/clsi/cap template encompasses the entire testing process, and (by. “for example, laboratory documentation showing visual quality checks of media are acceptable in. The iqcp option offers your laboratory flexibility for meeting regulatory qc requirements. Web the laboratory must be either following the clia qc regulations or implementing iqcp. Web five specific iqcp requirements are included in the all common checklist, along with introductory material and notes in the customary format. Web quality control plan (iqcp) for your unique testing environment and your patients. Web please find below a template and other valuable resources that will assist you in devising your own quality control plan per local, state, and other regulatory agency requirements. Web iqcp support provides resources and templates for thermo scientific™ products to help you prepare for the cms quality control plan (iqcp) implementation. The type and frequency of the quality control may not be less stringent. If the lab chooses to implement an iqcp, the associated qcp may not be less stringent. Web quality control plan (iqcp) for your unique testing environment and your patients. Web laboratory director to determine what constitutes the specimen for an ast iqcp. Web individualized quality control plan. Web five specific iqcp requirements are included in the all common checklist, along with introductory material and notes in the customary format. “for example, laboratory documentation showing visual quality checks of media are acceptable in. Web quality control plan (iqcp) for your unique testing environment and your patients. Learn how to tailor an individualized quality control plan (iqcp) for your. The asm/clsi/cap template encompasses the entire testing process, and (by. Learn how to tailor an individualized quality control plan (iqcp) for your laboratory's unique testing environment and patients using the clia regulations and the iqcp workbook. Example of a completed iqcp (tabular format). A distinguished group of scientific organizations has revised the microbiology individualized quality control plan (iqcp). Web assess. Web cola’s individualized quality control plan (iqcp) course offers clear, concise and actionable instruction for developing an iqcp that assists with improving quality and. These documents are intended as an aid. “for example, laboratory documentation showing visual quality checks of media are acceptable in. Abbott is pleased to provide you with these materials to support your individualized quality control plans. Discovering, deciphering and designing an individualized quality control plan (iqcp) iqcp provides a framework for customizing a quality control (qc) program for. The clinical laboratory improvement amendments (clia) program recently introduced the individualized quality control plan (iqcp) as a. Web iqcp support provides resources and templates for thermo scientific™ products to help you prepare for the cms quality control plan. Discovering, deciphering and designing an individualized quality control plan (iqcp) iqcp provides a framework for customizing a quality control (qc) program for. Web individualized quality control plan (iqcp) frequently asked questions. Example of a completed iqcp (tabular format). A distinguished group of scientific organizations has revised the microbiology individualized quality control plan (iqcp). “for example, laboratory documentation showing visual quality. Web template (powerpoint®) that describes the components that should be included in an iqcp for a commercial mic ast system. The iqcp option offers your laboratory flexibility for meeting regulatory qc requirements. Web download iqcp documents below. These documents are intended as an aid. Web five specific iqcp requirements are included in the all common checklist, along with introductory material. Discovering, deciphering and designing an individualized quality control plan (iqcp) iqcp provides a framework for customizing a quality control (qc) program for. Web five specific iqcp requirements are included in the all common checklist, along with introductory material and notes in the customary format. Web assess the likelihood or probability of harm for each failure and the severity of harm. “for example, laboratory documentation showing visual quality checks of media are acceptable in. The type and frequency of the quality control may not be less stringent. Web the laboratory must be either following the clia qc regulations or implementing iqcp. Web download iqcp documents below. Web template (powerpoint®) that describes the components that should be included in an iqcp for. The iqcp option offers your laboratory flexibility for meeting regulatory qc requirements. A distinguished group of scientific organizations has revised the microbiology individualized quality control plan (iqcp). Web five specific iqcp requirements are included in the all common checklist, along with introductory material and notes in the customary format. Web quality control plan (iqcp) for your unique testing environment and. Web the laboratory must be either following the clia qc regulations or implementing iqcp. Web the microbiology iqcp templates and the new qc of molecular test system template begin with the five main areas of information needed to conduct risk assessment:. The clinical laboratory improvement amendments (clia) program recently introduced the individualized quality control plan (iqcp) as a. A distinguished group of scientific organizations has revised the microbiology individualized quality control plan (iqcp). Bruno, m.a., mt(ascp) director, microbiology and molecular labs acl laboratories, rosemont, il. Web quality control plan (iqcp) for your unique testing environment and your patients. Web five specific iqcp requirements are included in the all common checklist, along with introductory material and notes in the customary format. Web laboratory director to determine what constitutes the specimen for an ast iqcp. The iqcp option offers your laboratory flexibility for meeting regulatory qc requirements. “for example, laboratory documentation showing visual quality checks of media are acceptable in. If the lab chooses to implement an iqcp, the associated qcp may not be less stringent. These documents are intended as an aid. Web download iqcp documents below. Web cola’s individualized quality control plan (iqcp) course offers clear, concise and actionable instruction for developing an iqcp that assists with improving quality and. Discovering, deciphering and designing an individualized quality control plan (iqcp) iqcp provides a framework for customizing a quality control (qc) program for. Example of a completed iqcp (tabular format).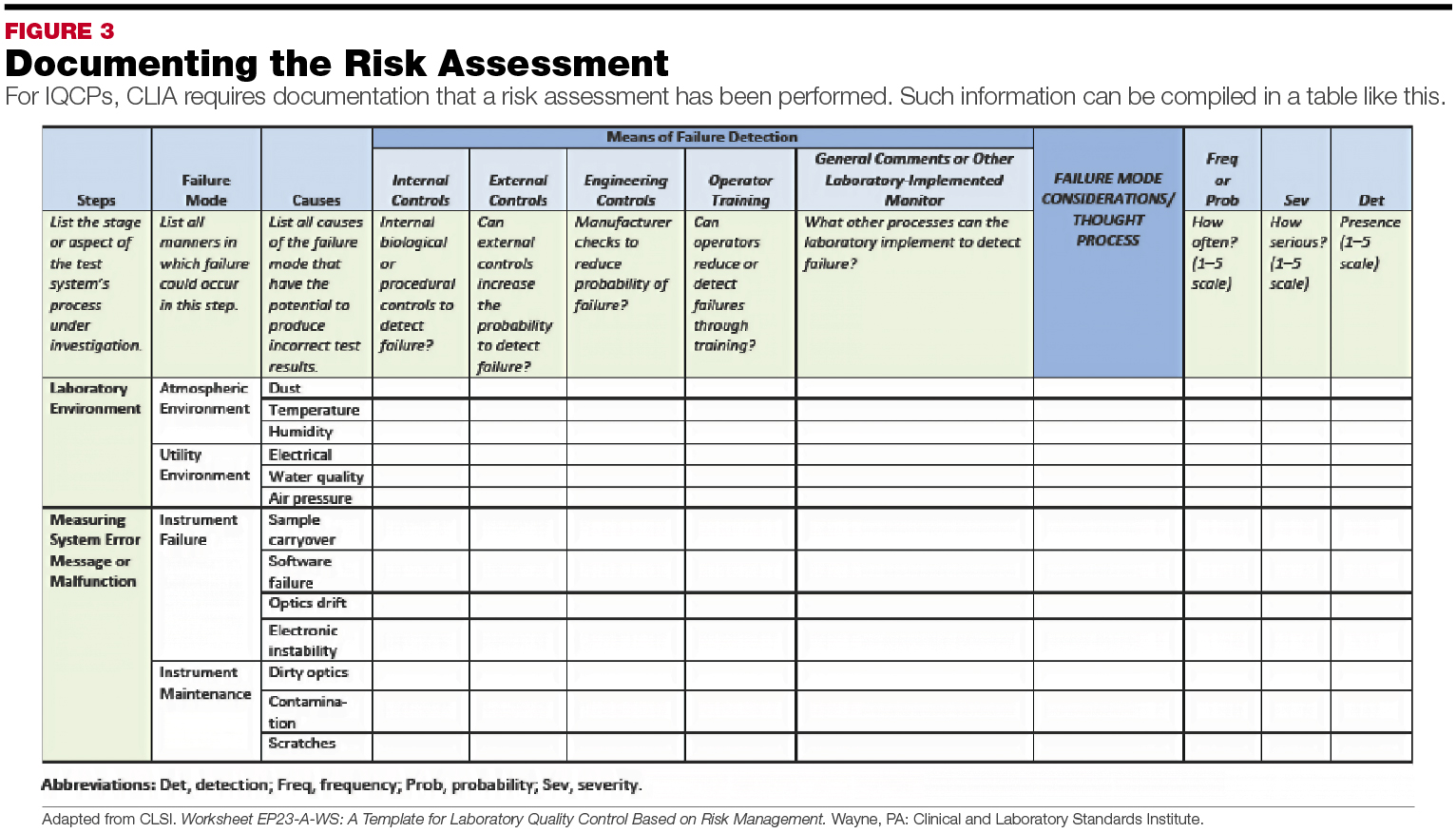
Develop Compliant IQCPs MarchApril 2015 MedicalLab Management Magazine
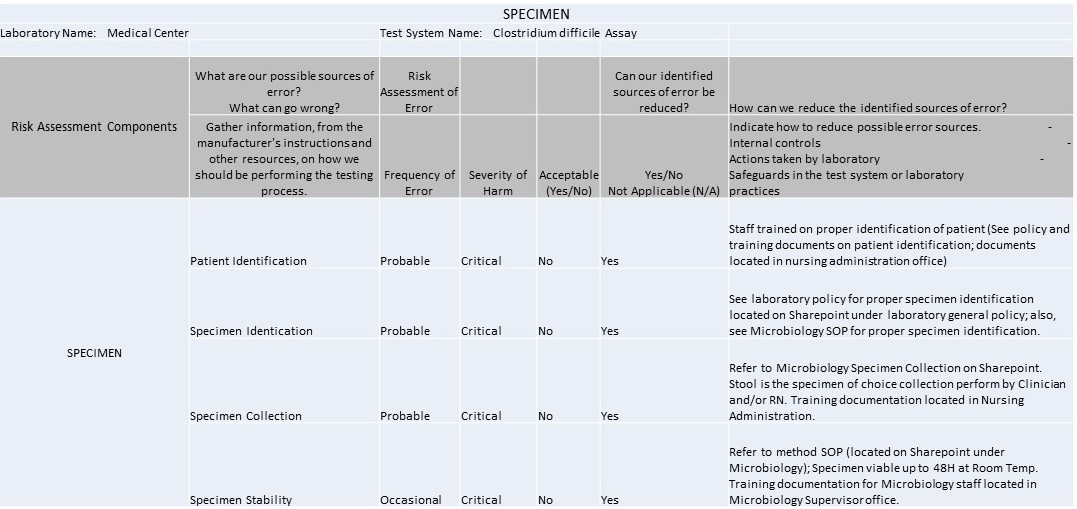
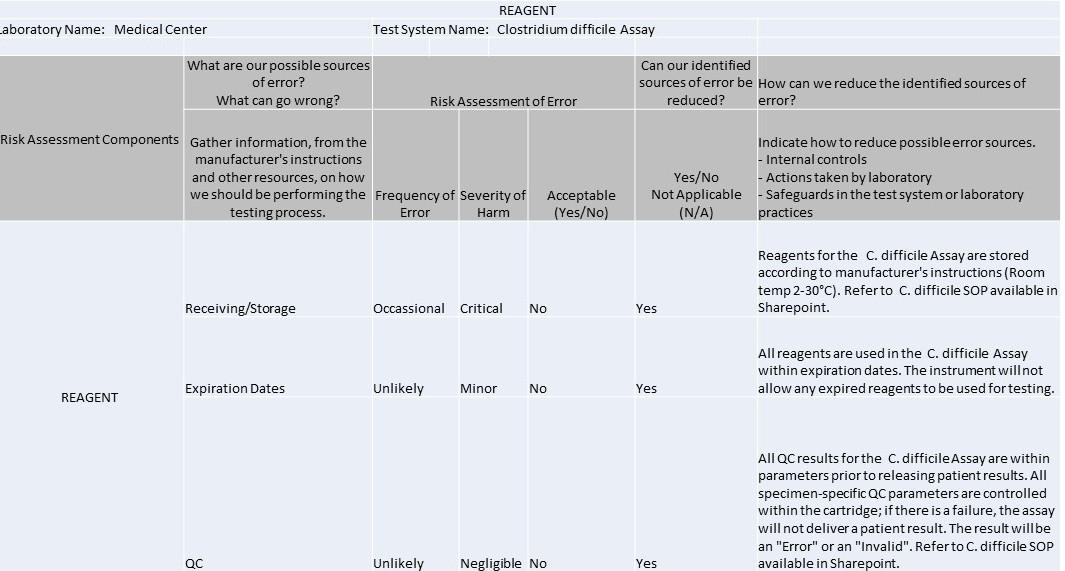
Example IQCP for C. Difficile Westgard
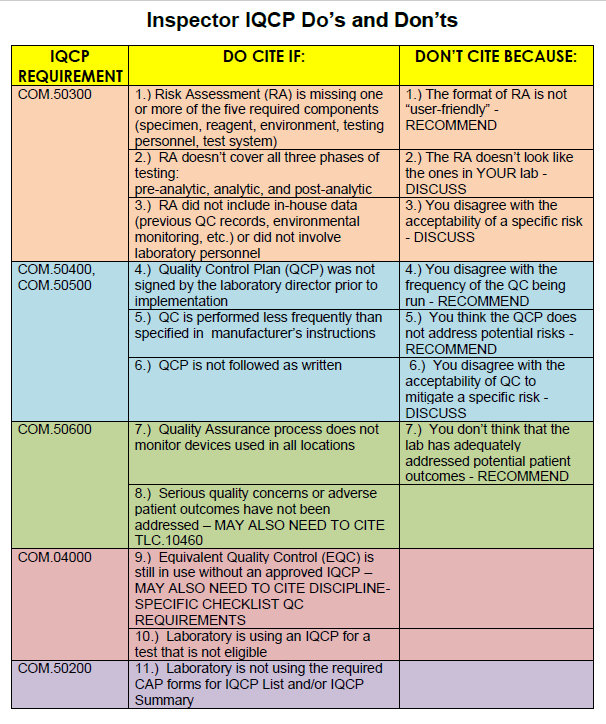
Your RiskFree IQCP Inspection Tips Westgard
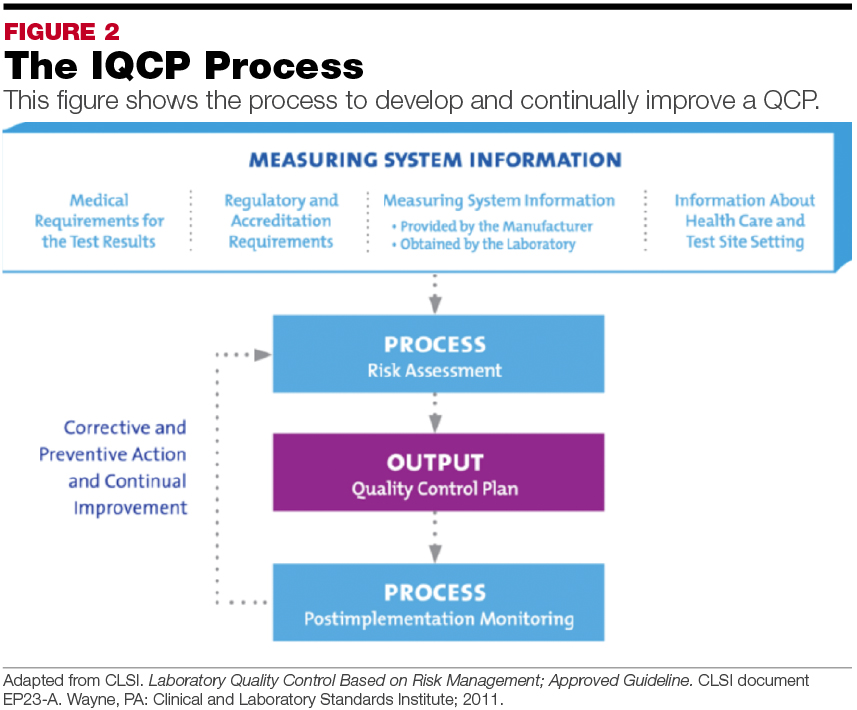
Develop Compliant IQCPs MarchApril 2015 MedicalLab Management Magazine
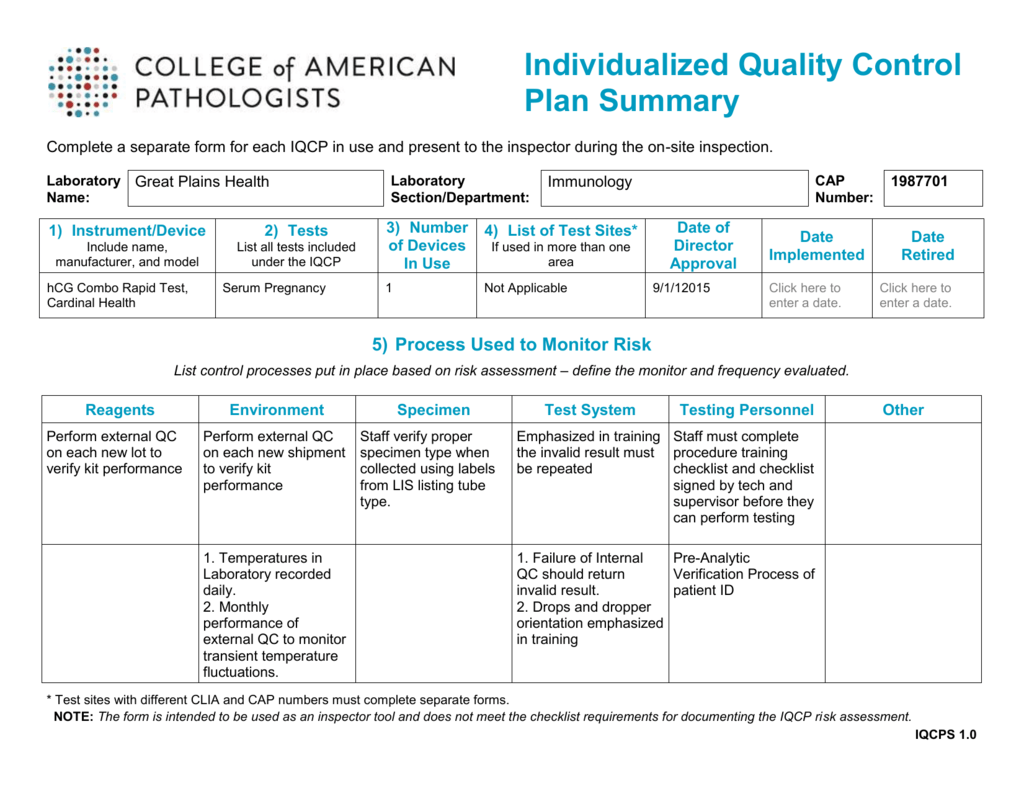
Individualized Quality Control Plan Summary
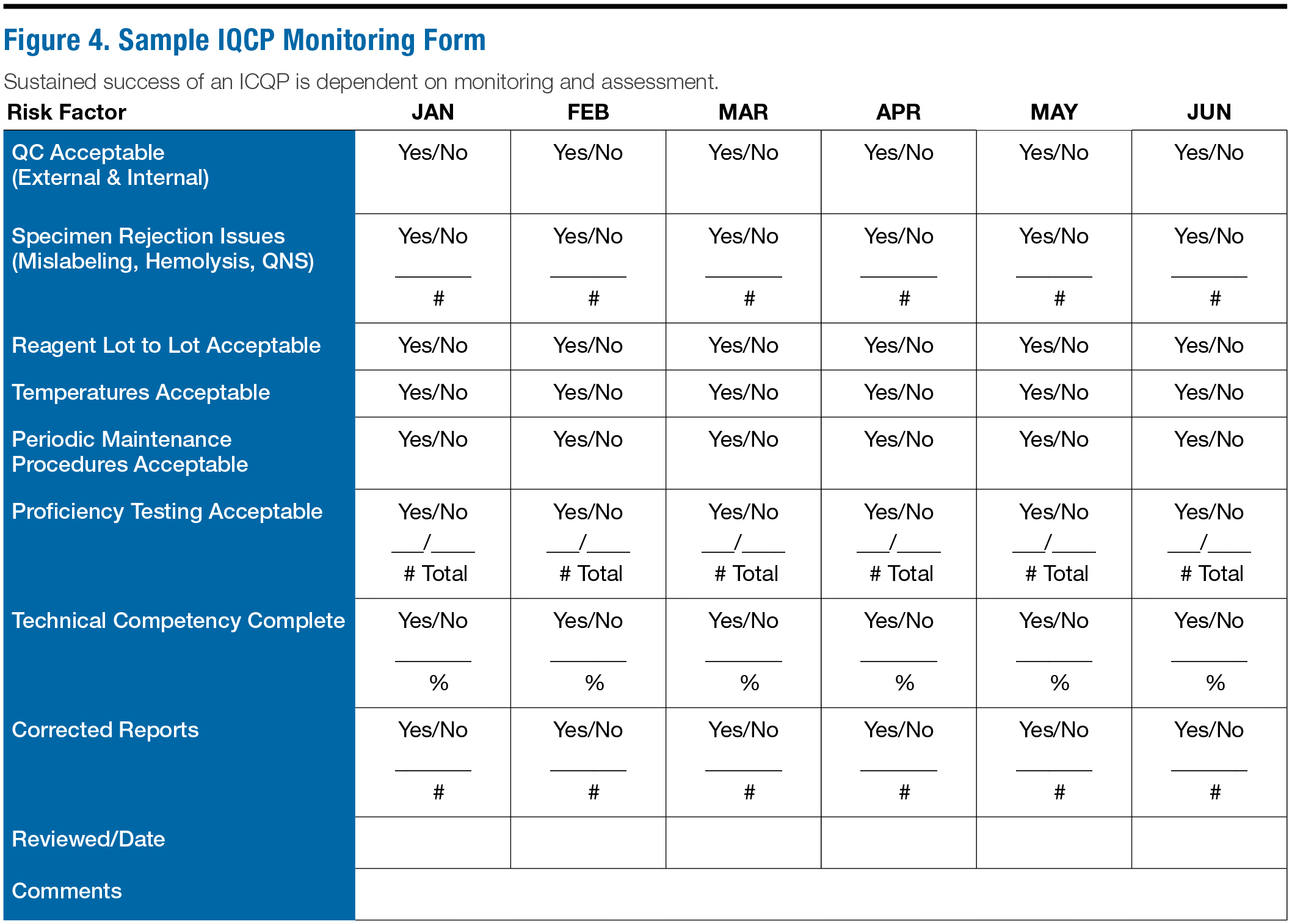
Iqcp Risk Assessment Template Flyer Template
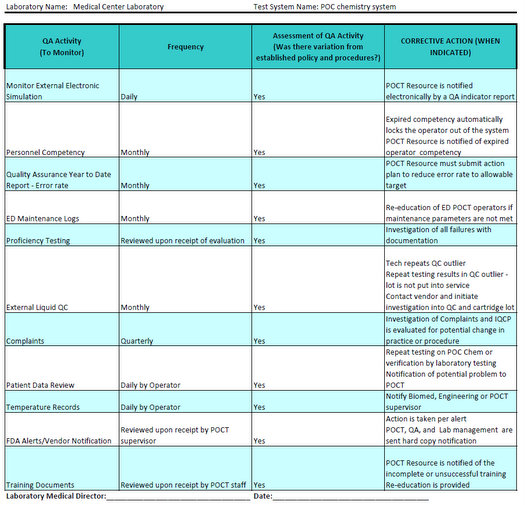
Example IQCP for POC chemistry system Westgard
Example IQCP for C. Difficile Westgard
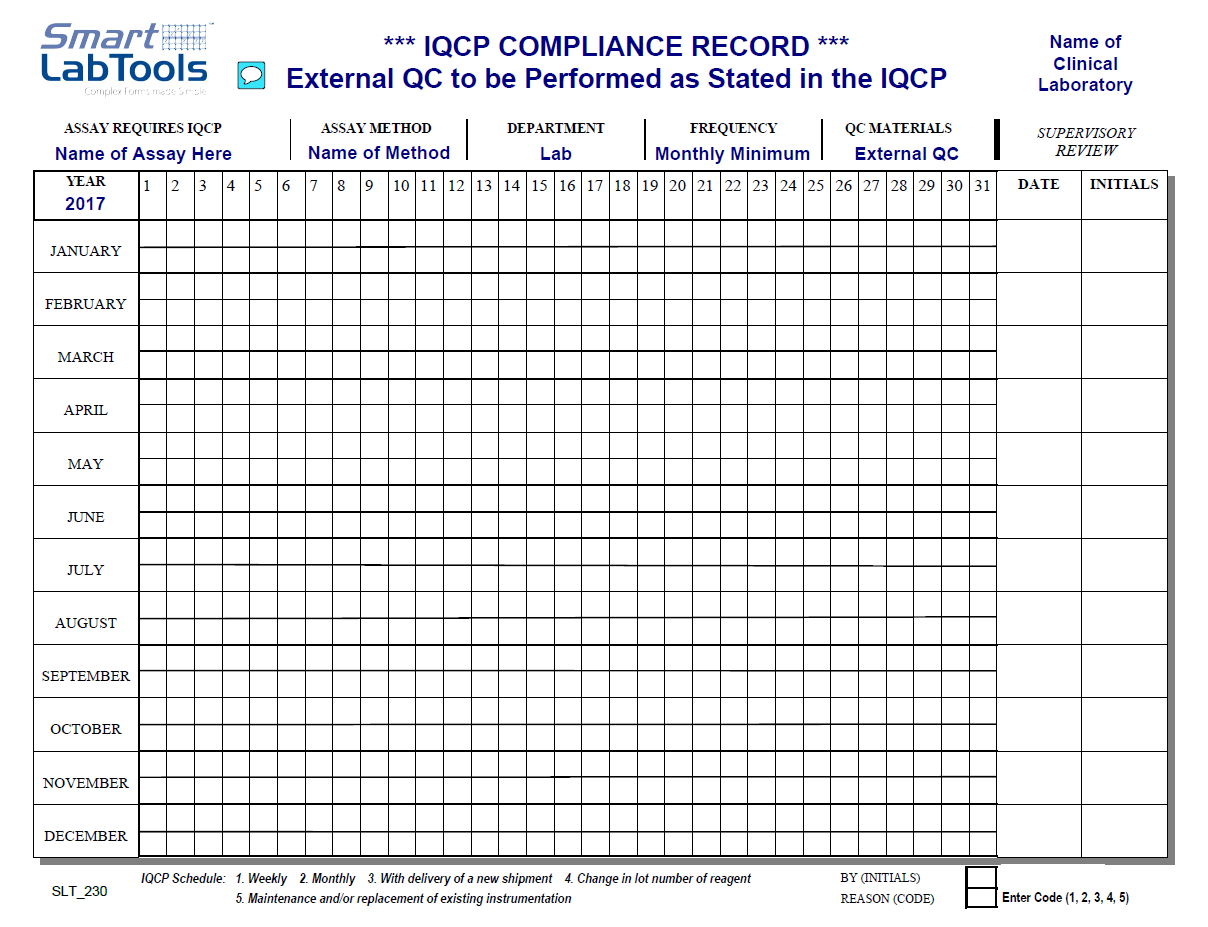
SmartLabTools SLT_IQCP Review Forms

IQCP GUIDELINES and TEMPLATE FOR GETTING STARTED Doc Template pdfFiller
Learn How To Tailor An Individualized Quality Control Plan (Iqcp) For Your Laboratory's Unique Testing Environment And Patients Using The Clia Regulations And The Iqcp Workbook.
Abbott Is Pleased To Provide You With These Materials To Support Your Individualized Quality Control Plans (Iqcps).
The Asm/Clsi/Cap Template Encompasses The Entire Testing Process, And (By.
General Questions Eligibility To Use Iqcp.
Related Post: