Irb Protocol Template
Irb Protocol Template - Web this section contains templates, forms, and guidance for studies in which the ohsu irb is waiving oversight to an external irb. A template used when submitting to neals irb. Additional irb templates are provided to promote transparency of irb operations. Document headerthe document header contains the h s d u w logo, the document type, and the document title. Under certain circumstances, it is recommended that researchers use a research protocol template in preparation for submitting their study via the new protocol submission xform in irbmanager.refer to the document below for. It is expected that the investigator will adapt the template to suit their. While these studies are likely to be minimal risk, they must include specific kinds of information to enable the irb to make the relevant regulatory determinations. The following protocol and consent templates are used by researchers in preparation for irb submission (see investigator manual for additional guidance on completing these documents). This part of the protocol deals specifically with existing data. This tool asks a series of questions to. Web •for ceded studies using a central irb (wcg/advarra/sterling irb), there will be a protocol available from the sponsor that you will use. The protocol must also comply with current fda electronic submission requirements. In the get started section, complete the following: Web the irb office has developed the following research protocol template for use by the nsu research community.. Choose a different pi, if necessary. This tool asks a series of questions to. Web irb application for human participant research use this form to submit a new human subject research protocol to the irb. The template guidance is only intended to help you draft the document and should not be retained in the final version. There are two templates. This form replaces the old adobe pdf form. The template guidance is only intended to help you draft the document and should not be retained in the final version. The irb toolkit’s registry and repository protocol template is the most efficient way for you to provide the information the irb needs. Common examples of research resources are a data or. A template used when submitting. Web visit tc irb’s website/how to submit/guides/writing for the irb for further tips. It is expected that the investigator will adapt the template to suit their. Web this section contains templates, forms, and guidance for studies in which the ohsu irb is waiving oversight to an external irb. Studies that will be reviewed by an. They follow the format of typical nih and industry multicenter protocols. Web post the version of the protocol that was originally approved and is relevant to the study at hand. Generally the purpose of a research resource is to help support. Every submission must have a new version date. Common examples of research resources are a data or biospecimen repository. Web •for ceded studies using a central irb (wcg/advarra/sterling irb), there will be a protocol available from the sponsor that you will use. This part of the protocol deals specifically with existing data. They follow the format of typical nih and industry multicenter protocols. Please consult the irb reliance webpage. This irb review application template is only to be used. Web the irb provides several protocol templates on this page. Web •for ceded studies using a central irb (wcg/advarra/sterling irb), there will be a protocol available from the sponsor that you will use. Remove all template guidance before you attach the file to the submission. Authorization to submit to neals irb (doc) 8/3/20: •for ceded studies where an external irb. Web the irb office has developed the following research protocol template for use by the nsu research community. The intervention template is ich gcp compliant. Hipaa and data use agreements are not part of the toolkit and can be found. Join the protocol navigation listserv subscribe. Web pasted as the summary of changes. In the get started section, complete the following: By using the ctep protocol template, the pi can ensure that the protocol submission will not be returned for. This part of the protocol deals specifically with existing data. Wcg authorization form (docx) 11/15/23: Web be met through other means. Web the irb provides several protocol templates on this page. Do not use it as a google doc or in a google drive; In the get started section, complete the following: It is expected that the investigator will adapt the template to suit their. How to name your documents. Web for a protocol not authored by cpws, ctep strongly urges the pi to use the protocol template provided with the full loi approval letter. They follow the format of typical nih and industry multicenter protocols. Studies that will be reviewed by an. The generic protocol template available on the protocol templates and guidelines page provides an example of how this should look. Web this section contains templates, forms, and guidance for studies in which the ohsu irb is waiving oversight to an external irb. The following protocol and consent templates are used by researchers in preparation for irb submission (see investigator manual for additional guidance on completing these documents). Authorization to submit to neals irb (doc) 8/3/20: Web the irb provides several protocol templates on this page. Assent templates and assent information. Under certain circumstances, it is recommended that researchers use a research protocol template in preparation for submitting their study via the new protocol submission xform in irbmanager.refer to the document below for. Please consult the irb reliance webpage. The natural history/observational protocol template, the repository protocol template, and the secondary research protocol template. Web the irb office has developed protocol templates for use by the northwestern university research community to describe research/human research activities. Wcg authorization form (docx) 11/15/23: The reliance agreement templates below, such as the iaa, are specific to ohsu waiving oversight. Submission to the hrpp for review of institutional requirements and irb for review for the approval criteria may require different documents.
Informed Consent Template Irb Master of Documents

IRB authorization agreement in Word and Pdf formats

Irb Protocol Template
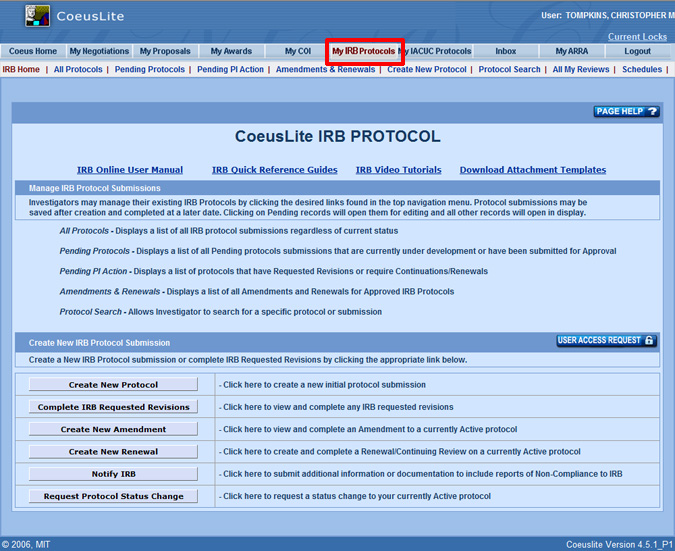
IRB Protocol Homepage Sponsored Program Services Coeus Purdue

Irb Protocol Template

IRB PROTOCOL REVIEW EXAMPLE GUIDE

How to Submit an IRB Protocol

example of an IRB Protocol

IRB Protocol Template Center for the Enhancement of Teaching

Protocol Amendment IRB Submission . Doc Template pdfFiller
A Template Used When Submitting To Neals Irb.
Human Subjects Protection Program/Irb 4
In The Get Started Section, Complete The Following:
Web Visit Tc Irb’s Website/How To Submit/Guides/Writing For The Irb For Further Tips.
Related Post: