Irb Template
Irb Template - As of may 17th, 2023, the icf templates available on our informed consent templates page replaces all previously released templates. National cancer institute (nci) / central institutional review board (cirb) usc required informed consent. Web this form is to be used for the following purposes: Web jhm irb combined informed consent/hipaa authorization template (march 2023, version 17): Web find protocol and consent templates for different types of research studies that require irb approval. Below are templates of commonly needed submission documents. Highlighting sample irb templates and submission documents. Web information sheet for exempt studies. Assent templates and assent information. For use when submitting new applications to be initially approved after. As of may 17th, 2023, the icf templates available on our informed consent templates page replaces all previously released templates. Sample templates and submission documents. The following protocol and consent templates are used by researchers in preparation for irb submission (see investigator manual (download) for. Web this form is to be used for the following purposes: Web the templates on. Web new for fall 2023: Find the new informed consent guidance resources here. Web investigators should use the protocol and consent templates that are available on the ohsrp website irb templates page. Assent templates and assent information. Web human subjects (irb) irb protocol submission; To register an irb if your institution or organization has not previously registered an irb. Below are templates of commonly needed submission documents. Web jhm irb combined informed consent/hipaa authorization template (march 2023, version 17): To aid in the development of research. Find the new informed consent guidance resources here. Web find protocol and consent templates for different types of research studies that require irb approval. To aid in the development of research. Assent templates and assent information. National cancer institute (nci) / central institutional review board (cirb) usc required informed consent. Web template name version number version date; Sop irb member standards and responsibilities. For use when submitting new applications to be initially approved after. Some ancillary reviews must be completed prior. Web template name version number version date; Web jhm irb combined informed consent/hipaa authorization template (march 2023, version 17): Find the new informed consent guidance resources here. To determine if you need to consent your subjects to be screened (in person, on the phone, or online), please see the section at. Web the irb review process and time vary depending on the level of review required. Web template name version number version date; Our forms and guidance documents are. To determine if you need to consent your subjects to be screened (in person, on the phone, or online), please see the section at. Sample templates and submission documents. Web find protocol and consent templates for different types of research studies that require irb approval. Highlighting sample irb templates and submission documents. Web this form is to be used for. Web jhm irb combined informed consent/hipaa authorization template (march 2023, version 17): Sop irb member standards and responsibilities. The following protocol and consent templates are used by researchers in preparation for irb submission (see investigator manual (download) for. To determine if you need to consent your subjects to be screened (in person, on the phone, or online), please see the. Web template name version number version date; Web the templates on this page are intended to help investigators construct documents that are as short as possible and written in plain language. As of may 17th, 2023, the icf templates available on our informed consent templates page replaces all previously released templates. Find the new informed consent guidance resources here. Web. Some ancillary reviews must be completed prior. Web find protocol and consent templates for different types of research studies that require irb approval. Web the irb review process and time vary depending on the level of review required. Web human subjects (irb) irb protocol submission; The following protocol and consent templates are used by researchers in preparation for irb submission. Sample templates and submission documents. Web new for fall 2023: Learn how to use the consent form wizard for educational and social. To determine if you need to consent your subjects to be screened (in person, on the phone, or online), please see the section at. As of may 17th, 2023, the icf templates available on our informed consent templates page replaces all previously released templates. To aid in the development of research. Assent templates and assent information. To update or renew the registration of. Find protocol templates for human research activities at northwestern university, such as biomedical, social and behavioral, and data and specimen analysis. The following protocol and consent templates are used by researchers in preparation for irb submission (see investigator manual (download) for. Web investigators should use the protocol and consent templates that are available on the ohsrp website irb templates page. To register an irb if your institution or organization has not previously registered an irb. Highlighting sample irb templates and submission documents. Web find templates for informed consent, assent, and debriefing forms for various types of human participant research studies. Web find protocol and consent templates for different types of research studies that require irb approval. It is recommended to use templates that incorporate.
IRB Proposal Template FIU Research

Research Pdf 10421 Cu Irb Flyer Template Flyer Template Word

Top 7 Irb Consent Form Templates free to download in PDF format

Form IRB2 Fill Out, Sign Online and Download Printable PDF, New

Request For Non Human Subject Research or IRB Exemption Doc Template
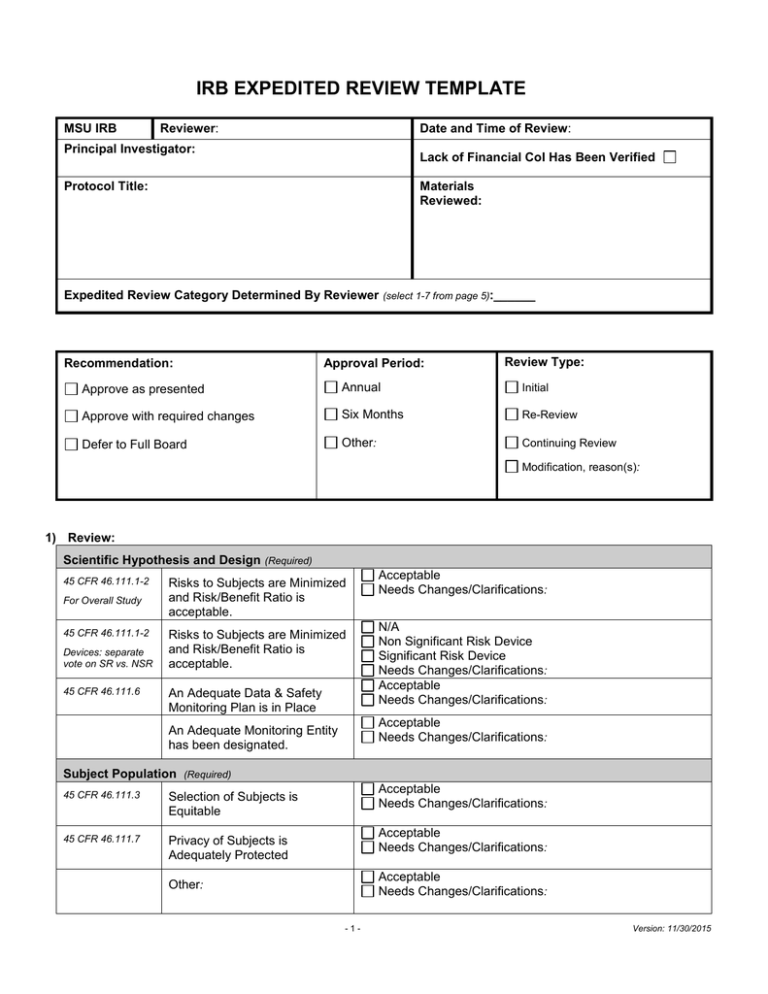
IRB EXPEDITED REVIEW TEMPLATE

Form IRB1 Fill Out, Sign Online and Download Printable PDF, New

28 Irb Form Templates free to download in PDF

Irb Template Form Fill Out and Sign Printable PDF Template airSlate

IRB authorization agreement in Word and Pdf formats
Some Ancillary Reviews Must Be Completed Prior.
Web The Irb Review Process And Time Vary Depending On The Level Of Review Required.
Web Human Subjects (Irb) Irb Protocol Submission;
National Cancer Institute (Nci) / Central Institutional Review Board (Cirb) Usc Required Informed Consent.
Related Post: