Paediatric Investigation Plan Template
Paediatric Investigation Plan Template - Web foremost among these are the electronic form for paediatric investigation plan application and request for waiver (a pdf file sometimes referred to as the‘pip template. Web paediatric investigation plan indication: Paediatric investigation plan (pip) and product specific waiver submissions to contain: Guideline on the format and content of applications for agreement or modification of a paediatric investigation plan. Templates, forms and submission dates. General approach to uk paediatric investigation plans. This common commentary addresses only the submission of an ipsp and pip for a drug or biological product for treatment or. Insights into research and practice for. Paediatric investigation plan (pip) and product specific waiver submissions to contain: Web preparing the paediatric investigation plan application adhd: General approach to uk paediatric investigation plans. Web this page lists the templates and forms required by companies wishing to apply for a paediatric investigation plan (pip), deferral or waiver. Web the templates for submission and submission deadlines can be found at: Initial pediatric study plan template. Web paediatric investigation plan indication: Guideline on the format and content of applications for agreement or modification of a paediatric investigation plan. Content of and process for submitting. Paediatric investigation plan (pip) and product specific waiver submissions to contain: This common commentary addresses only the submission of an ipsp and pip for a drug or biological product for treatment or. It is important to carefully. Templates, forms and submission dates. Web 1) define the pip strategy early in the writing process. Get emails about this page. Proposed indication in the paediatric population for the purpose of a pip, and at the time of submission of the pip, within a specific. • applications for which an ipsp is required • timing of an ipsp submission •. Web paediatric investigation plan (pip). Templates, forms and submission dates. Content of and process for submitting. Web the templates for submission and submission deadlines can be found at: • applications for which an ipsp is required • timing of an ipsp submission • content of an ipsp • content and timing of a. Web this page lists the templates and forms required by companies wishing to apply for a paediatric investigation plan (pip), deferral or waiver. Get emails about this page. It is important to carefully consider the most relevant condition and indication for your product in the. Initial pediatric study plan template. Web this guidance addresses the following: Web foremost among these are the electronic form for paediatric investigation plan application and request for waiver (a pdf file sometimes referred to as the‘pip template. Web paediatric investigation plan indication: Web 1) define the pip strategy early in the writing process. Web this guidance addresses the following: Web the templates for submission and submission deadlines can be found at: It is important to carefully consider the most relevant condition and indication for your product in the. General approach to uk paediatric investigation plans. Web application form (part a). Guideline on the format and content of applications for agreement or modification of a paediatric investigation plan. Web this guidance addresses the following: Web this page lists the templates and forms required by companies wishing to apply for a paediatric investigation plan (pip), deferral or waiver. Get emails about this page. Web this guidance addresses the following: Guideline on the format and content of applications for agreement or modification of a paediatric investigation plan. Web guideline on the format and content of applications. Web application form (part a). Paediatric investigation plan (pip) and product specific waiver submissions to contain: This common commentary addresses only the submission of an ipsp and pip for a drug or biological product for treatment or. Web this page lists the templates and forms required by companies wishing to apply for a paediatric investigation plan (pip), deferral or waiver.. Paediatric investigation plan (pip) and product specific waiver submissions to contain: Initial pediatric study plan template. Web 1) define the pip strategy early in the writing process. Web preparing the paediatric investigation plan application adhd: Web paediatric investigation plan indication: Web paediatric investigation plan (pip). Insights into research and practice for. Web this guidance addresses the following: Web the templates for submission and submission deadlines can be found at: Web foremost among these are the electronic form for paediatric investigation plan application and request for waiver (a pdf file sometimes referred to as the‘pip template. Templates, forms and submission dates. Web 1) define the pip strategy early in the writing process. Proposed indication in the paediatric population for the purpose of a pip, and at the time of submission of the pip, within a specific. Web paediatric investigation plan indication: Paediatric investigation plan (pip) and product specific waiver submissions to contain: Web this page lists the templates and forms required by companies wishing to apply for a paediatric investigation plan (pip), deferral or waiver. Get emails about this page. Web preparing the paediatric investigation plan application adhd: • applications for which an ipsp is required • timing of an ipsp submission • content of an ipsp • content and timing of a. General approach to uk paediatric investigation plans. This common commentary addresses only the submission of an ipsp and pip for a drug or biological product for treatment or.
Planning your Paediatric Investigation Plan (PIP) Submission in Euro…

Planning your Paediatric Investigation Plan (PIP) Submission in Euro…

Fillable Online Paediatric investigation plans Templates, forms and

PPT Regulatory Requirements for Orphan Drugs Delivery PowerPoint

Planning your Paediatric Investigation Plan (PIP) Submission in Euro…
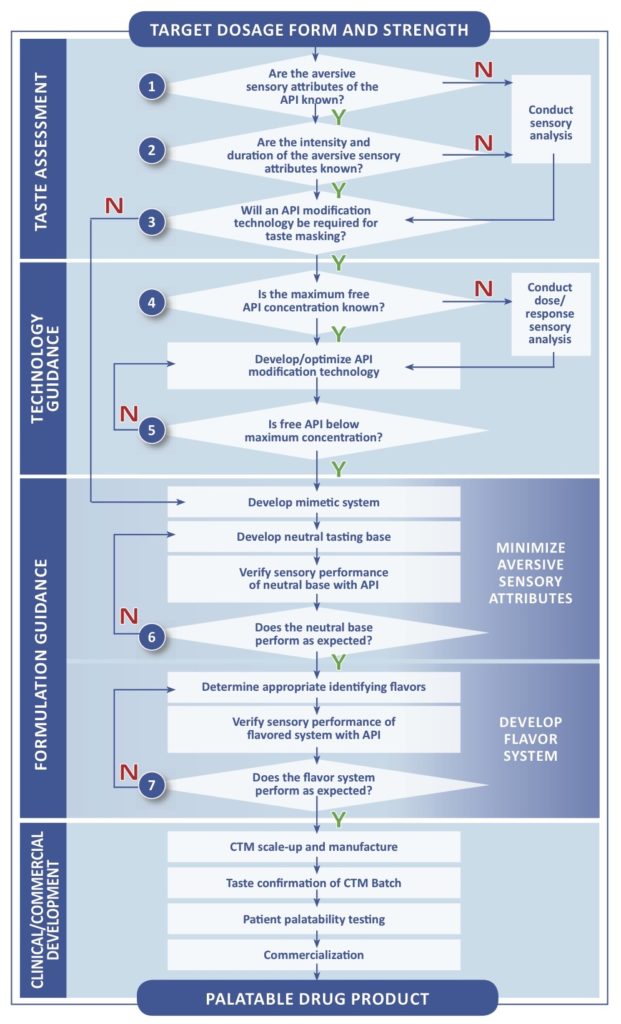
Paediatric Investigation Plan Template
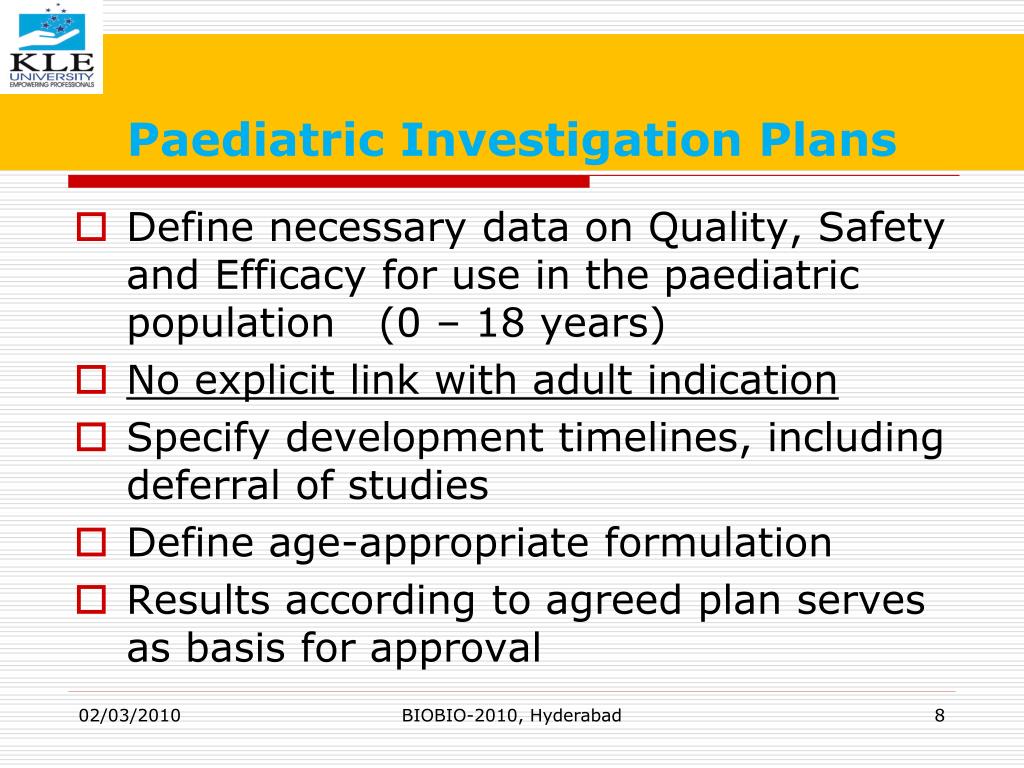
PPT Regulatory Requirements for Orphan Drugs Delivery PowerPoint
Pediatric Care Plan Template Medical Diagnosis Health Care
Paediatric Investigation Plan Template
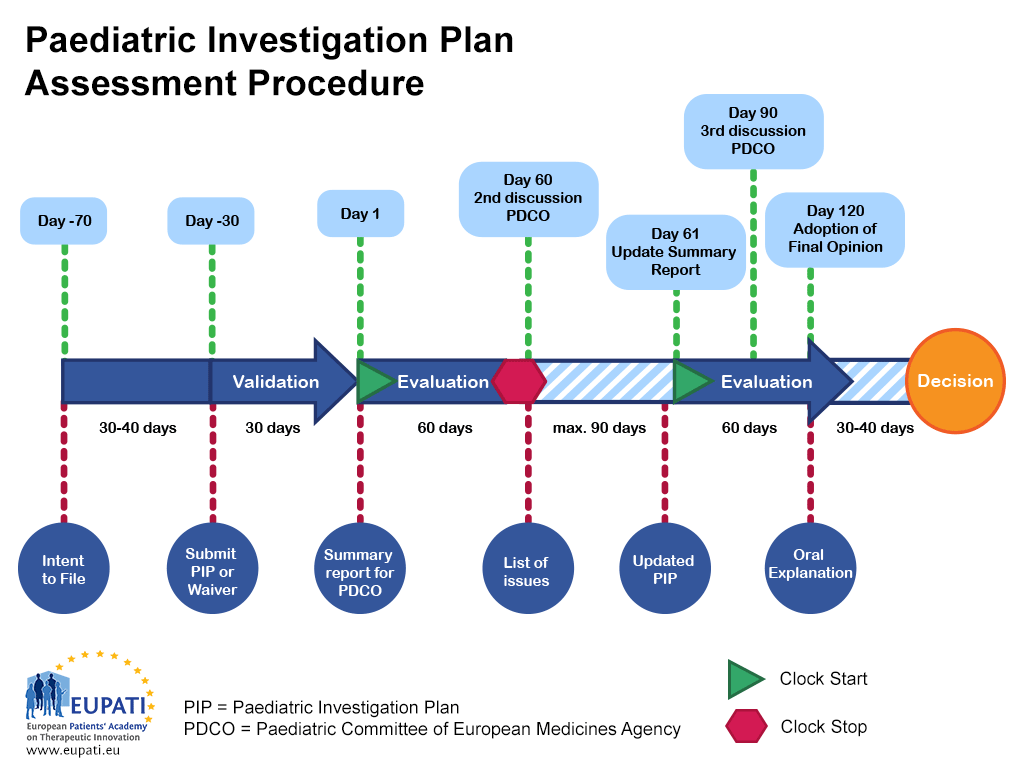
Paediatric medicine Paediatric Investigation Plan EUPATI Toolbox
Content Of And Process For Submitting.
It Is Important To Carefully Consider The Most Relevant Condition And Indication For Your Product In The.
Guideline On The Format And Content Of Applications For Agreement Or Modification Of A Paediatric Investigation Plan.
Web Application Form (Part A).
Related Post:
