Protocol Template
Protocol Template - Web the following protocol templates are available to assist you in developing a standalone protocol: Learn how to use them, when to submit them, and what. Web listed below are several templates to assist you in composing your protocol document. Web learn how to write a protocol for clinical research studies, including different types of design and templates. For biomedical clinical investigations evaluating drugs and/or devices, the. Web the ich m11 guideline provides a harmonised template and technical specification for clinical protocols to facilitate data exchange between regulatory. Web a research protocol is a detailed study design or set of instructions for carrying out a specific experimental process or procedure. Web find protocol templates and forms for biomedical and social behavioral research at northwestern university. Many funders such as the nhs health research authority. Web a study protocol is an important document that specifies the research plan for a clinical study. Web a research protocol must start from the definition of the coordinator of the whole study: Web learn how to write and develop clinical trial protocols that comply with regulatory and ethical standards. Web learn how to write a protocol for clinical research studies, including different types of design and templates. None of the templates are likely to be. Web. Web the pulp and paper tool offers a collection of tools that cover the emission sources typically associated with a pulp and paper plant. Web find protocol templates and forms for biomedical and social behavioral research at northwestern university. Web download a microsoft word document with all 51 spirit headings and item identifiers for structuring study protocols for randomised trials.. Web the following protocol templates are available to assist you in developing a standalone protocol: Find guidelines, checklists and examples to follow for. Web a research protocol must start from the definition of the coordinator of the whole study: Web pasted as the summary of changes. Download the draft guidance document. Find sample protocol templates and resources from. Many funders such as the nhs health research authority. For biomedical clinical investigations evaluating drugs and/or devices, the. Learn how to use them, when to submit them, and what. Web a study protocol is an important document that specifies the research plan for a clinical study. Download the draft guidance document. Bishop mr, dickinson m, purtill d, et al. Learn how to use them, when to submit them, and what. Web the following protocol templates are available to assist you in developing a standalone protocol: Web learn how to write a protocol for clinical research studies, including different types of design and templates. Web the following protocol templates are available to assist you in developing a standalone protocol: Download the draft guidance document. Web learn how to write a protocol for clinical research studies, including different types of design and templates. All the details of the main investigator must be reported in the first paragraph. Web listed below are several templates to assist. Learn how to use them, when to submit them, and what. Many funders such as the nhs health research authority. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted. Web the ich m11 guideline provides a harmonised template and technical. Find guidelines, checklists and examples to follow for. Web this page includes seven different protocol templates for developing a variety of different new research protocols. Web pasted as the summary of changes. Bishop mr, dickinson m, purtill d, et al. Instructions specific to items on the templates appear in red text in brackets. Download the draft guidance document. Web the ich m11 guideline provides a harmonised template and technical specification for clinical protocols to facilitate data exchange between regulatory. Clinical electronic structured harmonised protocol december 2022. It is a joint product of the international council of. Many funders such as the nhs health research authority. Download the draft guidance document. Web a research protocol is a detailed study design or set of instructions for carrying out a specific experimental process or procedure. Web find protocol templates and forms for biomedical and social behavioral research at northwestern university. For biomedical clinical investigations evaluating drugs and/or devices, the. Web learn how to write a protocol for clinical. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted. Web a research protocol must start from the definition of the coordinator of the whole study: Learn how to use them, when to submit them, and what. Web the following protocol templates are available to assist you in developing a standalone protocol: Web a research protocol is a detailed study design or set of instructions for carrying out a specific experimental process or procedure. Instructions specific to items on the templates appear in red text in brackets. Web the pulp and paper tool offers a collection of tools that cover the emission sources typically associated with a pulp and paper plant. Generic protocol documents and instructions for ctep studies. Web suggested templates for phase 1 and 2 clinical trials. Learn how to use the template and. Web find protocol templates and forms for biomedical and social behavioral research at northwestern university. Web find protocol templates for descriptive, observational and intervention studies that follow the format of typical nih and industry multicenter protocols. Web the cap cancer protocols provide guidelines for collecting the essential data elements for complete reporting of malignant tumors and optimal patient care. Download the draft guidance document. It is a joint product of the international council of. Web a study protocol is an important document that specifies the research plan for a clinical study.
Research protocol template

Free Microsoft Word Standard Operating Procedure Sop Templates ZOHAL
qualitative research protocol example Archives Digital Documents
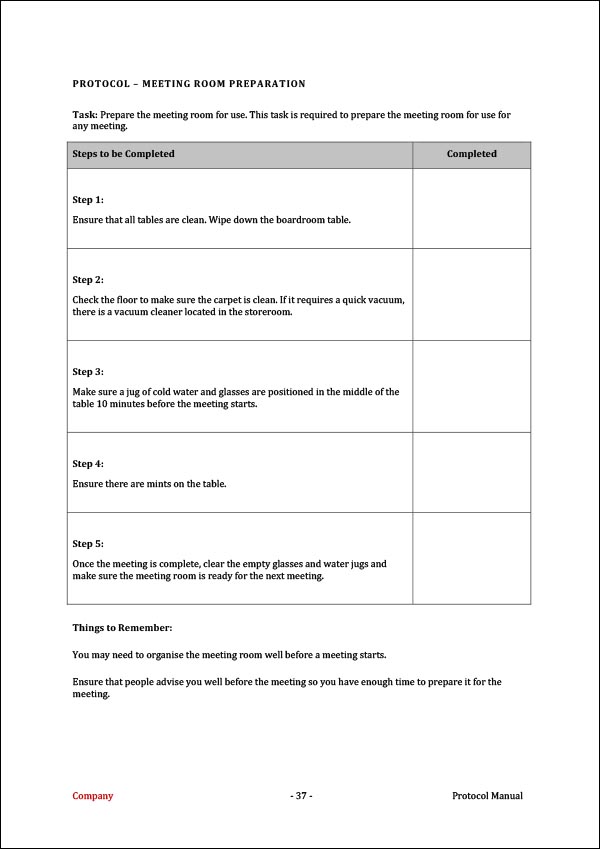
Meeting Protocol Template PDF Template

Protocol Template Treatment Use
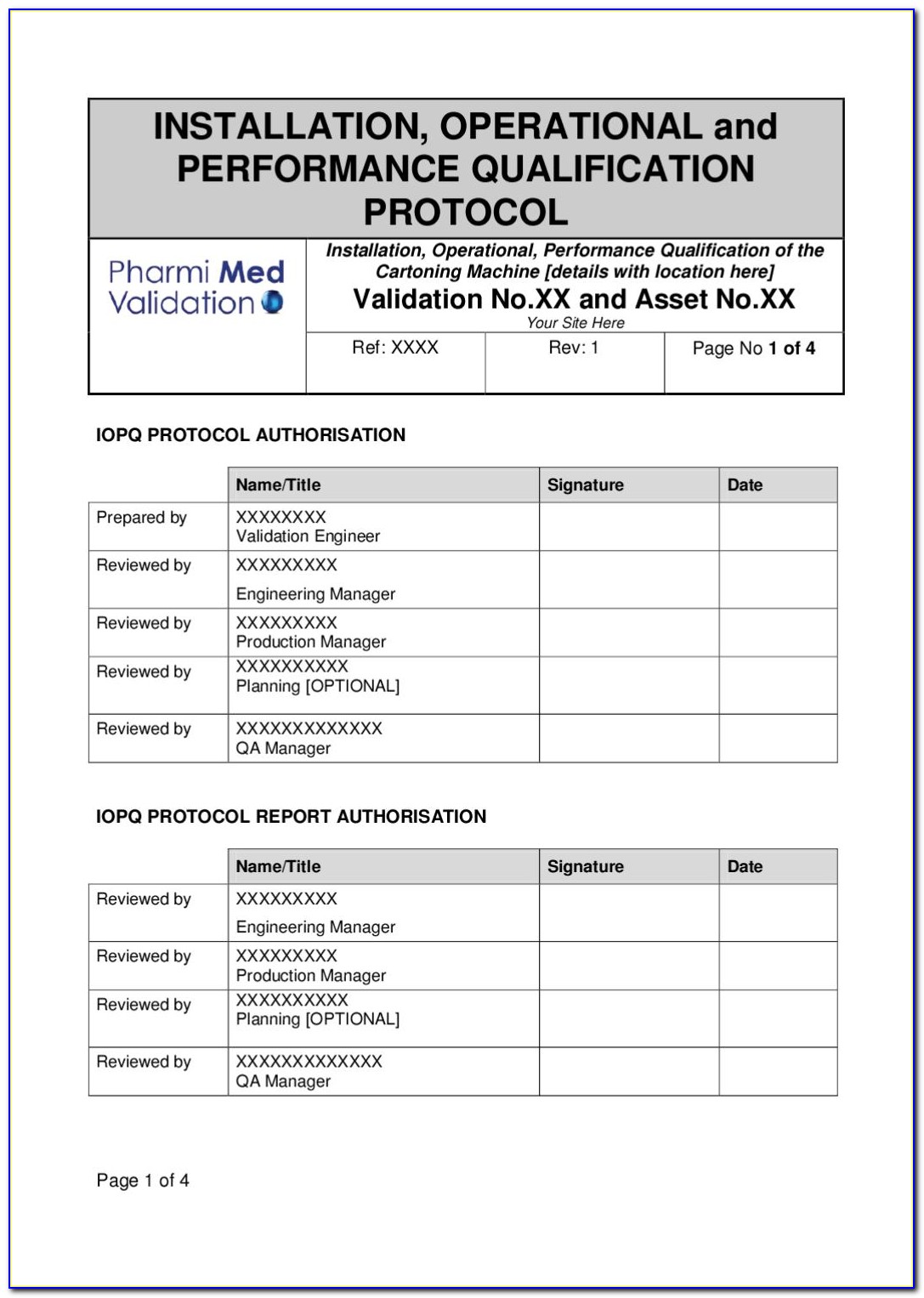
Iq Oq Protocol Template

Testing Protocol Template

KHP CTU Protocol Template
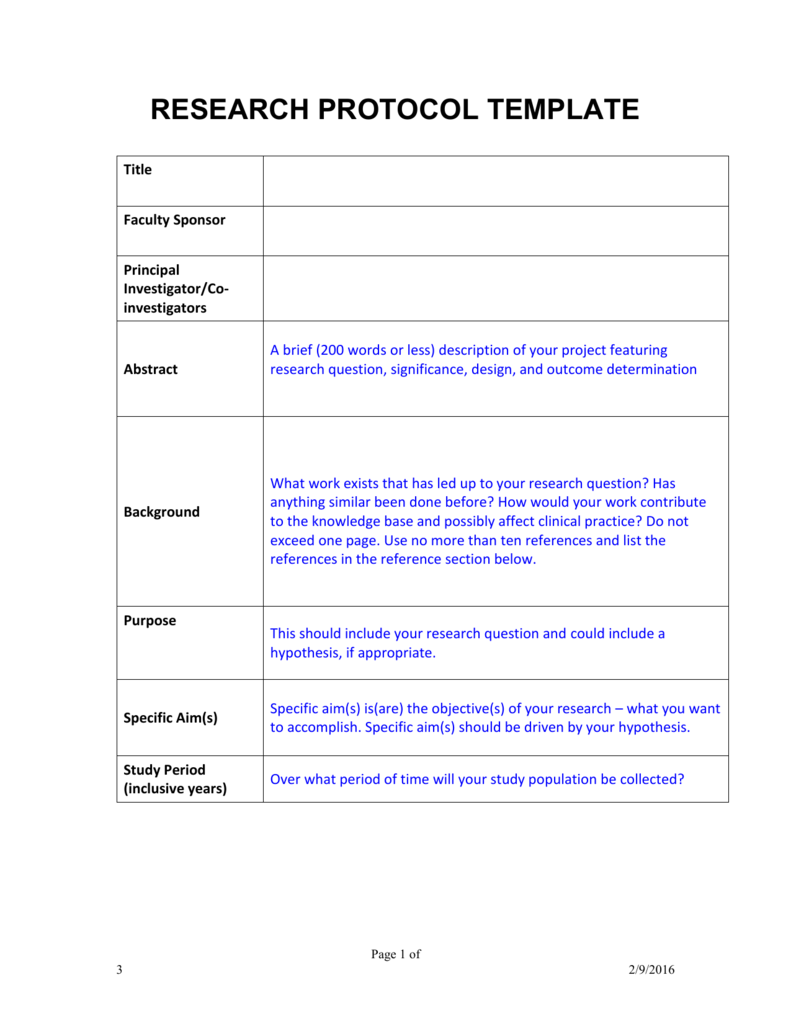
research protocol template

Protocol Template Biomedical
Web This Page Includes Seven Different Protocol Templates For Developing A Variety Of Different New Research Protocols.
Many Funders Such As The Nhs Health Research Authority.
Web Listed Below Are Several Templates To Assist You In Composing Your Protocol Document.
Web Learn How To Write A Protocol For Clinical Research Studies, Including Different Types Of Design And Templates.
Related Post: