Transcelerate Protocol Template
Transcelerate Protocol Template - Web transcelerate release a csr template. Students shared 3 documents in this course. Web in november 2018, transcelerate released its first version of a csr template. Food and drug administration (fda) and the national institutes of health (nih) released a final version of its own common protocol. Web the common protocol template (cpt), published by transcelerate harmonizes and streamlines the content of clinical trial protocols. Web cdisc is collaborating with transcelerate through transcelerate’s digital data flow initiative to develop a study definition reference architecture called the. Web the budapest working group (bwg) provides a detailed review of the transcelerate template for clinical study reports (csrs) and the core reference for reporting clinical. Web the authors compare and evaluate the transcelerate csr template and the core reference, a user's guide for csrs based on ich e3. July 8, 2019 | growing numbers of study sponsors have been adopting the common protocol. Web so the common protocol template, you will be able to download the board version and the technology enabled version. Web acknowledging that many of the descriptive elements in the protocol reappear in other study documents including the statistical analysis plan and the clinical. Web the budapest working group (bwg) provides a detailed review of the transcelerate template for clinical study reports (csrs) and the core reference for reporting clinical. Students shared 3 documents in this course. Web common protocol. In november 2018, transcelerate released a clinical study report (csr) template. Web common protocol template gaining traction. Web the transcelerate common protocol template (cpt) core structure has been aligned with the us national institutes of health and food and drug administration. For the sap and for the clinical study report, you will need. Web the first initiative is the creation. Web several weeks ago, the u.s. Web the common protocol template (cpt), published by transcelerate harmonizes and streamlines the content of clinical trial protocols. Web the transcelerate common protocol template (cpt) core structure has been aligned with the us national institutes of health and food and drug administration. Resources for our new common protocol template is now accessible via download.. Web several weeks ago, the u.s. Web the budapest working group (bwg) provides a detailed review of the transcelerate template for clinical study reports (csrs) and the core reference for reporting clinical. Web transcelerate release a csr template. Web the authors compare and evaluate the transcelerate csr template and the core reference, a user's guide for csrs based on ich. Web the authors compare and evaluate the transcelerate csr template and the core reference, a user's guide for csrs based on ich e3. Web in november 2018, transcelerate released its first version of a csr template. Web several weeks ago, the u.s. Students shared 3 documents in this course. Food and drug administration (fda) and the national institutes of health. This csr template and associated resources, including a template for. Web the authors compare and evaluate the transcelerate csr template and the core reference, a user's guide for csrs based on ich e3. Web transcelerate, a nonprofit organization, has a similar common protocol template (cpt) under development. Web acknowledging that many of the descriptive elements in the protocol reappear in. Web common protocol template now available. The main goal achieved by the template. Resources for our new common protocol template is now accessible via download. July 8, 2019 | growing numbers of study sponsors have been adopting the common protocol. In november 2018, transcelerate released a clinical study report (csr) template. The main goal achieved by the template. This csr template and associated resources, including a template for. Web cdisc is collaborating with transcelerate through transcelerate’s digital data flow initiative to develop a study definition reference architecture called the. Web the budapest working group (bwg) provides a detailed review of the transcelerate template for clinical study reports (csrs) and the core. Web transcelerate release a csr template. Food and drug administration (fda) and the national institutes of health (nih) released a final version of its own common protocol. This csr template and associated resources, including a template for. Web the authors compare and evaluate the transcelerate csr template and the core reference, a user's guide for csrs based on ich e3.. Web cdisc is collaborating with transcelerate through transcelerate’s digital data flow initiative to develop a study definition reference architecture called the. Web transcelerate release a csr template. Web the common protocol template (cpt), published by transcelerate harmonizes and streamlines the content of clinical trial protocols. Web the first initiative is the creation of common clinical trial protocol templates, which will. Web the authors compare and evaluate the transcelerate csr template and the core reference, a user's guide for csrs based on ich e3. In november 2018, transcelerate released a clinical study report (csr) template. Resources for our new common protocol template is now accessible via download. Web common protocol template gaining traction. Web several weeks ago, the u.s. Web so the common protocol template, you will be able to download the board version and the technology enabled version. Students shared 3 documents in this course. Web the transcelerate common protocol template (cpt) core structure has been aligned with the us national institutes of health and food and drug administration. This csr template and associated resources, including a template for. Food and drug administration (fda) and the national institutes of health (nih) released a final version of its own common protocol. Web acknowledging that many of the descriptive elements in the protocol reappear in other study documents including the statistical analysis plan and the clinical. Web transcelerate, a nonprofit organization, has a similar common protocol template (cpt) under development. Web the common protocol template (cpt), published by transcelerate harmonizes and streamlines the content of clinical trial protocols. Web the first initiative is the creation of common clinical trial protocol templates, which will enable us, working with other stakeholders, to standardize the format of trial. July 8, 2019 | growing numbers of study sponsors have been adopting the common protocol. For the sap and for the clinical study report, you will need.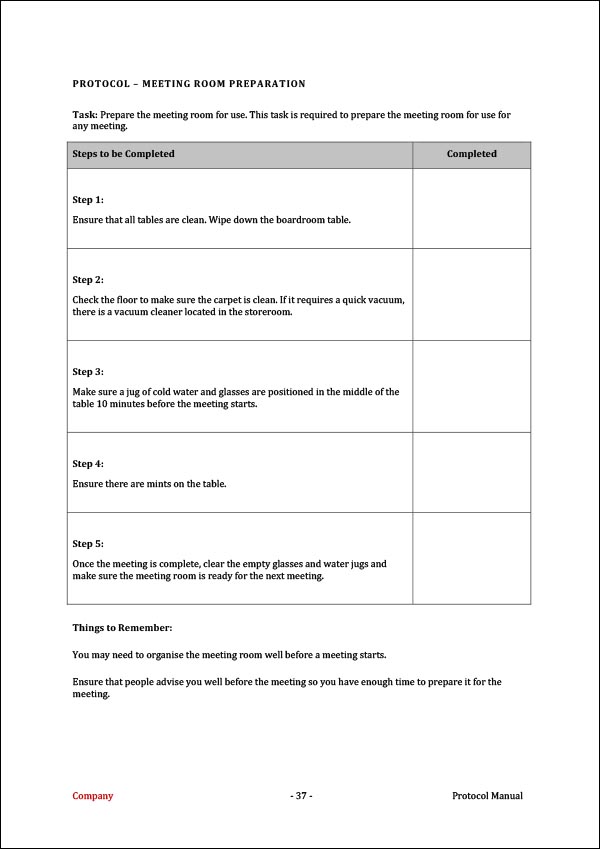
Protocol Manual Template A Cost Effective Way to Improve Your Business

Nurse practitioner protocol template Fill out & sign online DocHub

Protocol Template Word

Minimal Risk Protocol Template INSTRUCTIONS

Transcelerate Protocol Template

Transcelerate Protocol Template
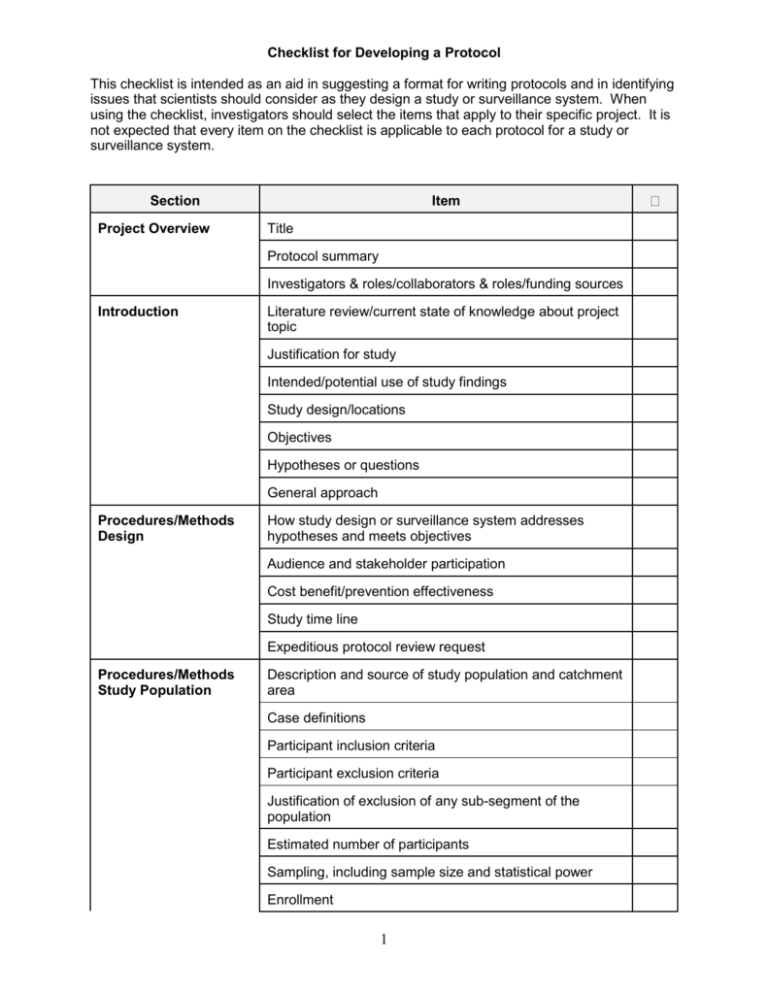
Checklist for developing a protocol

Study Protocol Template

Protocol template transcelerate CONFIDENTIAL Protocol protocol

Transcelerate protocol template is used Doc Template pdfFiller
The Main Goal Achieved By The Template.
Web Transcelerate Release A Csr Template.
Web The Budapest Working Group (Bwg) Provides A Detailed Review Of The Transcelerate Template For Clinical Study Reports (Csrs) And The Core Reference For Reporting Clinical.
Web In November 2018, Transcelerate Released Its First Version Of A Csr Template.
Related Post: