Validation Master Plan Template
Validation Master Plan Template - Home › complianceonline standards › fda validation › validation master plan template. 5.2.7 for large projects involving many materials, a materials. You can create a great protocol, using a template. The purpose of the validation master plan is to document the compliance requirements for the site and to. Fast, easy & securepaperless workflowpaperless solutionsfree mobile app Web a validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation activities within a pharmaceutical or medical device. Web validation master plan [iso 13485 templates] iso 13485 / mdr document template: The template includes a requirements traceability. Web the validation master plan: Web master validation plan is a strategic document which identifies the elements to be validated, the approach to be taken for validation of each element, the organizational. Web master validation plan is a strategic document which identifies the elements to be validated, the approach to be taken for validation of each element, the organizational. Web a validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation activities within a pharmaceutical or medical device. Define the product and process flow ii.. Web three (3) options to create a validation master plan. A free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all. Web master validation plan is a strategic document which identifies the elements to be validated, the approach to be taken for validation of each element, the organizational. Web validation master plan [iso. The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and acceptance. You can create a great protocol, using a template. The purpose of the validation master plan is to document the compliance requirements for the site and to. Senior gxp regulatory compliance expert. Web the validation master plan is. Web the validation master plan is a summary of validation strategy. Web 2.2 scope of the document. The purpose of the validation master plan is to document the compliance requirements for the site and to. Web a quick validation master plan checklist. You can create a great protocol, using a template. The purpose of this document is to record the schedule for. Whether you're setting out to develop a vmp or seek to identify weaknesses in an existing one, the following questions can serve as a. You can download a free sample of a validation master. Web a quick validation master plan checklist. Web what is validation master plan (vmp): Web the validation master plan is a summary of validation strategy. Guide, methods, tools and template. Fast, easy & securepaperless workflowpaperless solutionsfree mobile app This template is a tool for creating a customized plan for validating a product, system, or process. Define the product and process flow ii. The template includes a requirements traceability. Web the validation master plan: Web critical components of a vmp. Tips for writing a validation master plan. Web a validation master plan, also referred to as “vmp”, outlines the principles involved in the qualification of a facility, defining the areas and systems to be validated, and provides a. Web master validation plan is a strategic document which identifies the elements to be validated, the approach to be taken for validation of each element, the organizational. Guide, methods, tools and template. Web a validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation activities within a pharmaceutical or medical device. Senior gxp. Fast, easy & securepaperless workflowpaperless solutionsfree mobile app Define the product and process flow ii. Web critical components of a vmp. Web validation master plan template. Web master validation plan is a strategic document which identifies the elements to be validated, the approach to be taken for validation of each element, the organizational. Facilitate fda inspections with a validation master plan. Web at the core of the validation process is a fundamental document known as a validation master plan (vmp). The purpose of this document is to record the schedule for. Web a validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation activities within a. Web a validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation activities within a pharmaceutical or medical device. Define the product and process flow ii. January 22, 2024 by caitlin o'donnell. 2.2.1 it is considered that the principles defined in the individual recommendation documents can be applied equally in the manufacture of active. Web a quick validation master plan checklist. Web 2.2 scope of the document. You can create a great protocol, using a template. Web critical components of a vmp. The purpose of this document is to record the schedule for. Web the validation master plan is a summary of validation strategy. Web the validation master plan: This template is a tool for creating a customized plan for validating a product, system, or process. Web download a free template to plan and execute verification and validation activities for software development projects. Web validation master plan [iso 13485 templates] iso 13485 / mdr document template: Web validation master plan template. The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and acceptance.
How to create a Validation Master Plan in 5 steps. Templates & more
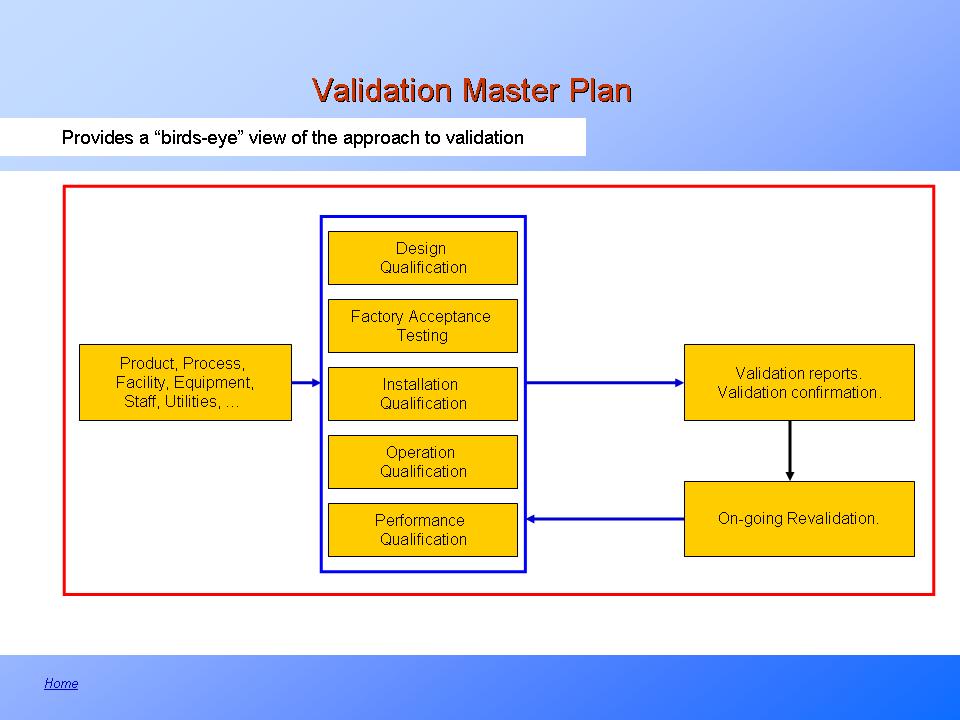
Overview of the Validation Master Plan PresentationEZE

Validation Master Plan Template Validation Center
Validation Master Plan Bio Chem Shop

How To Create A Validation Master Plan In 5 Steps Tem vrogue.co

FREE 9+ Sample Validation Plan Templates in PDF MS Word
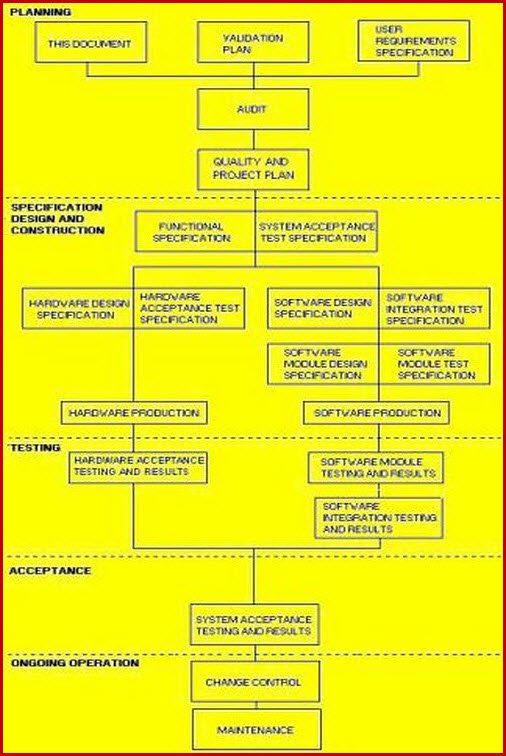
Validation Master Plan (VMP) Downloadable Interactive Template.

FREE 9+ Sample Validation Plan Templates in PDF MS Word
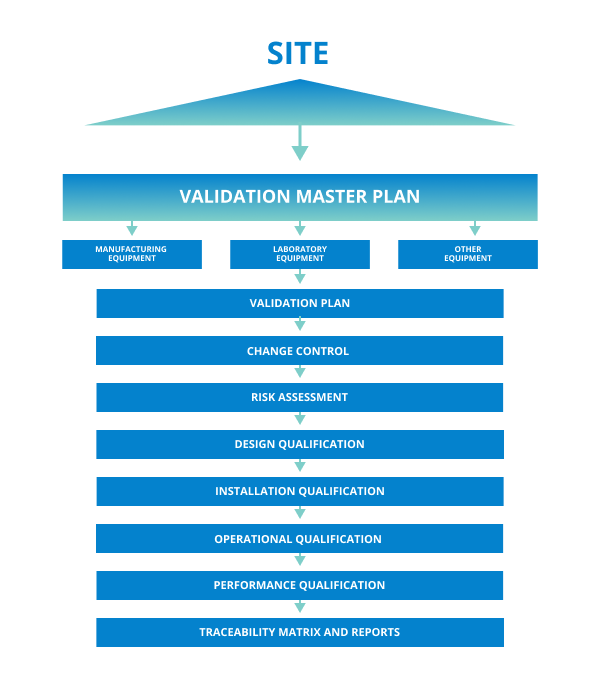
Validation Master Plan Egnyte
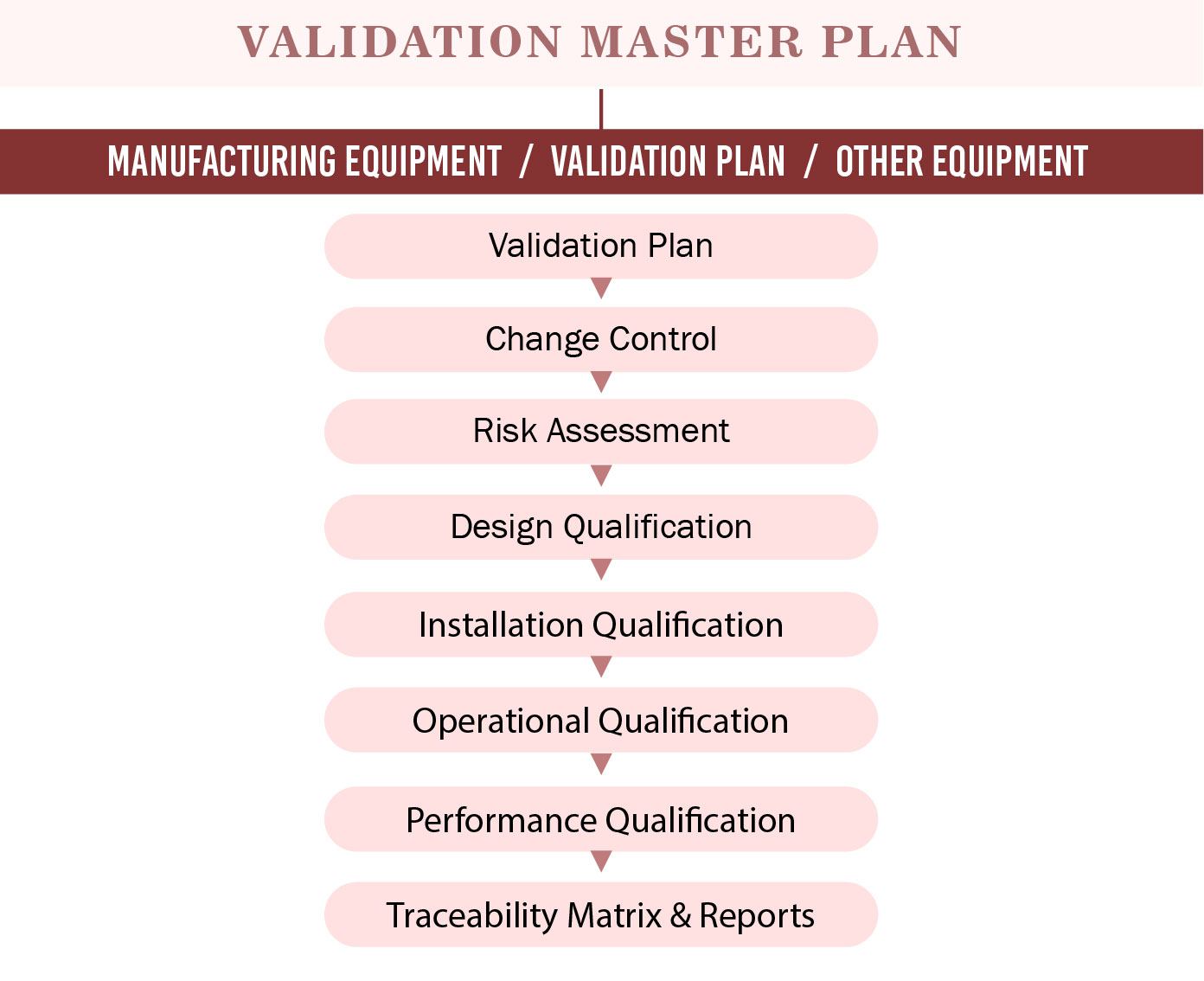
Validation Master Plan Template
Tips For Writing A Validation Master Plan.
Do You Have Strict Requirements For Quality And Safety, With.
You Can Download A Free Sample Of A Validation Master.
5.2.7 For Large Projects Involving Many Materials, A Materials.
Related Post: