Vmp Template
Vmp Template - Web the purpose of this validation master plan (hereinafter known as the “plan” or “vmp”) is to provide guidelines and protocol for the validation of applicable processes, equipment,. Web the validation master plan considering the above, we can now complete the validation program adage: Web (validation master plan) or lower tier documentation alone may cover the qualification of materials. The document is fully editable so that you can adapt it to your company design. The validation master plan is a summary of validation strategy. Web in this comprehensive guide, we’ll address key questions such as “what does a validation master plan include?” and “how can i create a vmp?” and “who is responsible for. The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and. Web validation templates design is of critical importance when regulatory compliance and clarity of purpose are prerequisites. Web validation master plan individual validation plan manufacturers often choose to develop a validation master plan (vmp) as a tool to control and monitor the status of validation. 5.2.8 the headings of the vmp may be based on the above list. Tell them what you’re going to do (vmp). Web validation master plan individual validation plan manufacturers often choose to develop a validation master plan (vmp) as a tool to control and monitor the status of validation. Web what is validation master plan (vmp): Web a validation master plan (vmp) is a documented plan that outlines the overall strategy and approach. Web the validation master plan considering the above, we can now complete the validation program adage: The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and. Web in this comprehensive guide, we’ll address key questions such as “what does a validation master plan include?” and “how can i create. The validation master plan is a summary of validation strategy. Web validation master plan template document control details this will include details such as vmp reference number, version number, date and authorisation signatures. Web a validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation activities within a pharmaceutical or medical device. Documents. In these circumstances it is only the bold and confident that. Web master validation plan is a strategic document which identifies the elements to be validated, the approach to be taken for validation of each element, the. Tell them what you’re going to do (vmp). The validation master plan serves as a roadmap that helps to set the course, justifying. Tell them what you’re going to do (vmp). 5.2.8 the headings of the vmp may be based on the above list. Web a validation master plan (vmp), a segment of gmps (good manufacturing practices) for pharmaceutical, biotech and medical device organizations, is a report that. The objective of this document is to outline the validation plan for a gmp site. Tell them what you’re going to do (vmp). Web a validation master plan, also referred to as “vmp”, outlines the principles involved in the qualification of a facility, defining the areas and systems to be validated, and provides a. Web validation master plans discuss validation activities across an entire site or within an organization. Web validation master plan individual validation. Web a validation master plan (vmp), a segment of gmps (good manufacturing practices) for pharmaceutical, biotech and medical device organizations, is a report that. Documents include placeholder marks for all. 5.2.8 the headings of the vmp may be based on the above list. Web the validation master plan considering the above, we can now complete the validation program adage: Web. The objective of this document is to outline the validation plan for a gmp site and to ensure that all the. Web validation templates design is of critical importance when regulatory compliance and clarity of purpose are prerequisites. The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and. Web. Web preview validation master plan template. Web in this comprehensive guide, we’ll address key questions such as “what does a validation master plan include?” and “how can i create a vmp?” and “who is responsible for. Web validation master plan template document control details this will include details such as vmp reference number, version number, date and authorisation signatures. 5.2.8. Web a validation master plan (vmp), a segment of gmps (good manufacturing practices) for pharmaceutical, biotech and medical device organizations, is a report that. Web validation templates design is of critical importance when regulatory compliance and clarity of purpose are prerequisites. Web what is validation master plan (vmp): In these circumstances it is only the bold and confident that. Web. Web a validation master plan, also referred to as “vmp”, outlines the principles involved in the qualification of a facility, defining the areas and systems to be validated, and provides a. Web validation master plan individual validation plan manufacturers often choose to develop a validation master plan (vmp) as a tool to control and monitor the status of validation. Web validation templates design is of critical importance when regulatory compliance and clarity of purpose are prerequisites. Web in this comprehensive guide, we’ll address key questions such as “what does a validation master plan include?” and “how can i create a vmp?” and “who is responsible for. Web a validation master plan (vmp), a segment of gmps (good manufacturing practices) for pharmaceutical, biotech and medical device organizations, is a report that. The objective of this document is to outline the validation plan for a gmp site and to ensure that all the. The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and. The document is fully editable so that you can adapt it to your company design. Web what is validation master plan (vmp): Web validation master plans discuss validation activities across an entire site or within an organization. 5.2.8 the headings of the vmp may be based on the above list. Web master validation plan is a strategic document which identifies the elements to be validated, the approach to be taken for validation of each element, the. Tell them what you’re going to do (vmp). Web the purpose of this validation master plan (hereinafter known as the “plan” or “vmp”) is to provide guidelines and protocol for the validation of applicable processes, equipment,. Web validation master plan template document control details this will include details such as vmp reference number, version number, date and authorisation signatures. Web a validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation activities within a pharmaceutical or medical device.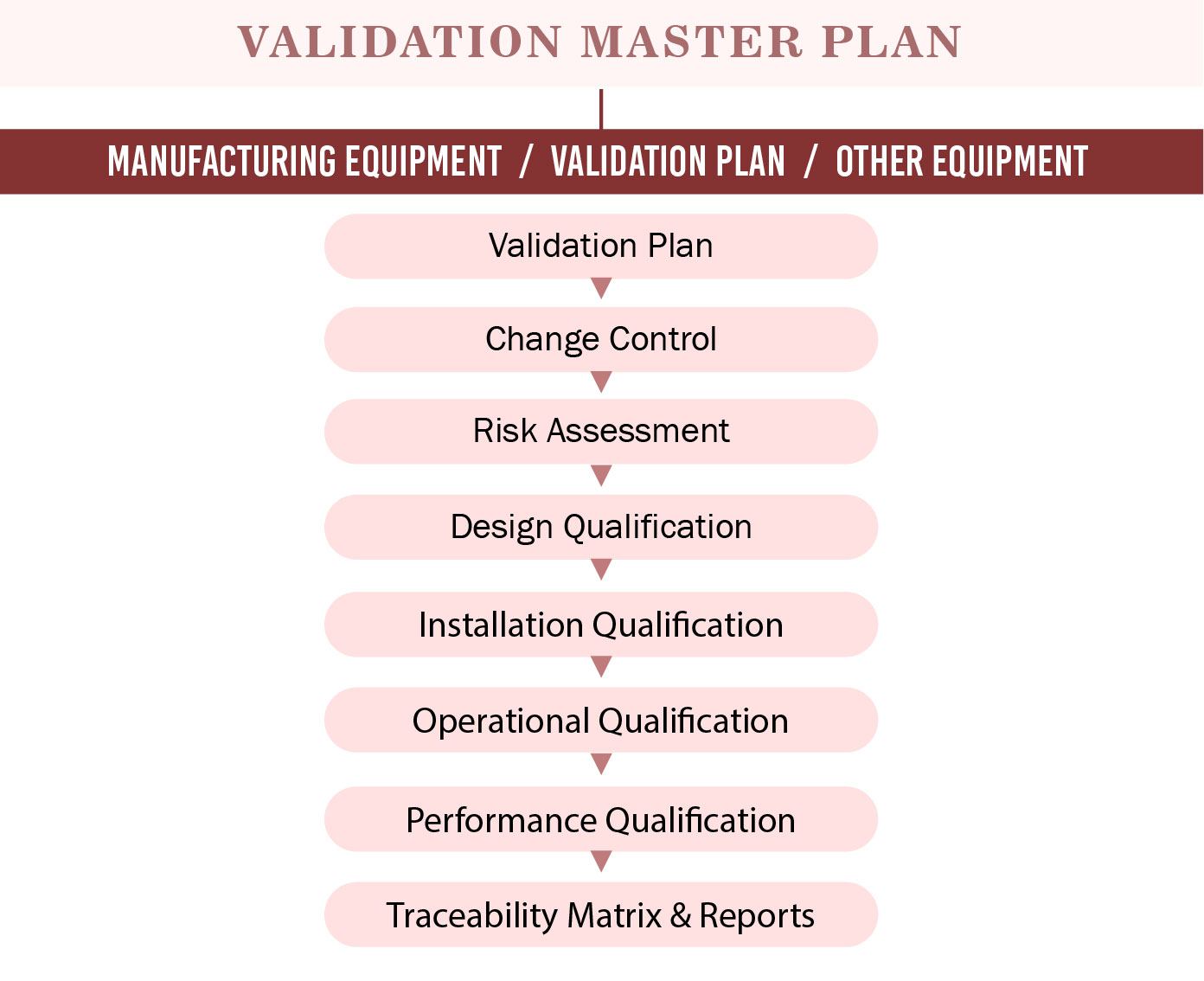
Medical Device Validation Master Plan (VMP) Consultancy Firm Operon

10+ Validation Plan Templates Sample Templates

Validation Master Plan. Understand the importance and benefits
VMP Template PDF Verification And Validation Business Process
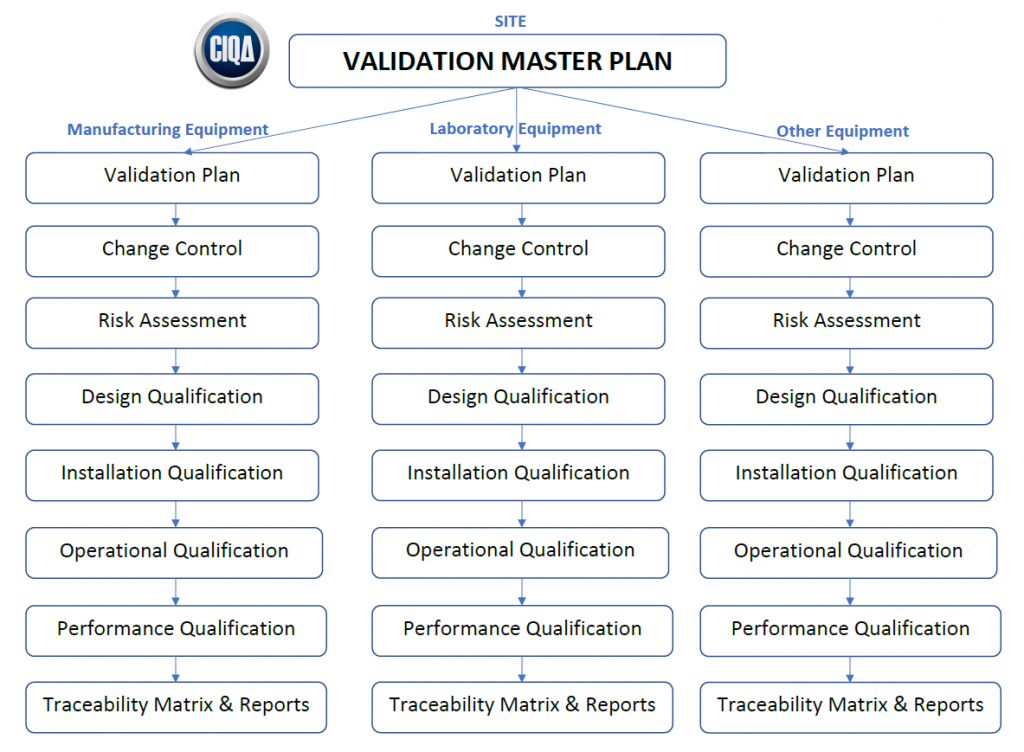
Overview of the Validation Master Plan PresentationEZE
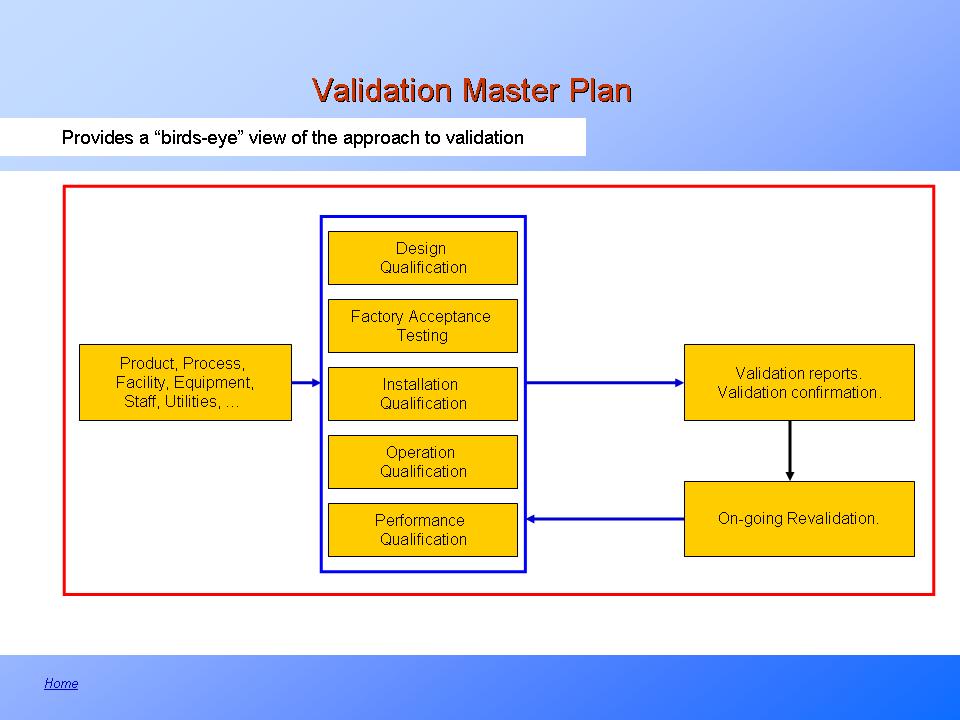
Validation Master Plan (VMP) Downloadable Interactive Template.
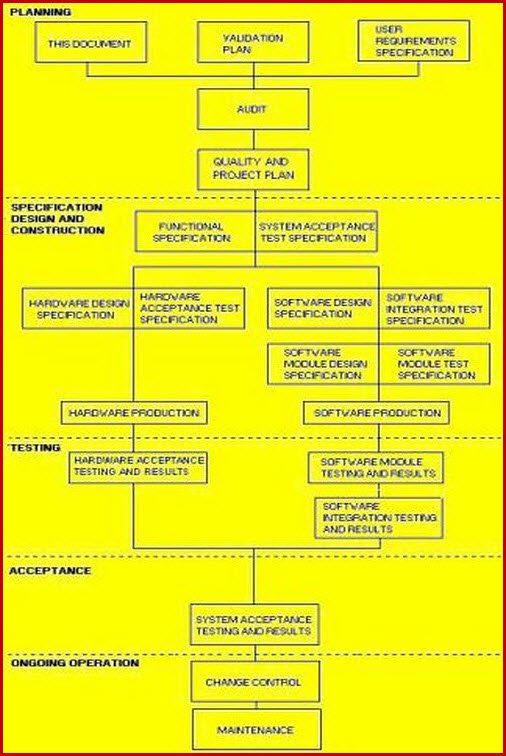
How to create a Validation Master Plan in 5 steps. Templates & more

Validation Master Plan Bio Chem Shop

Validation Master Plan (VMP) Template LexDocPharma
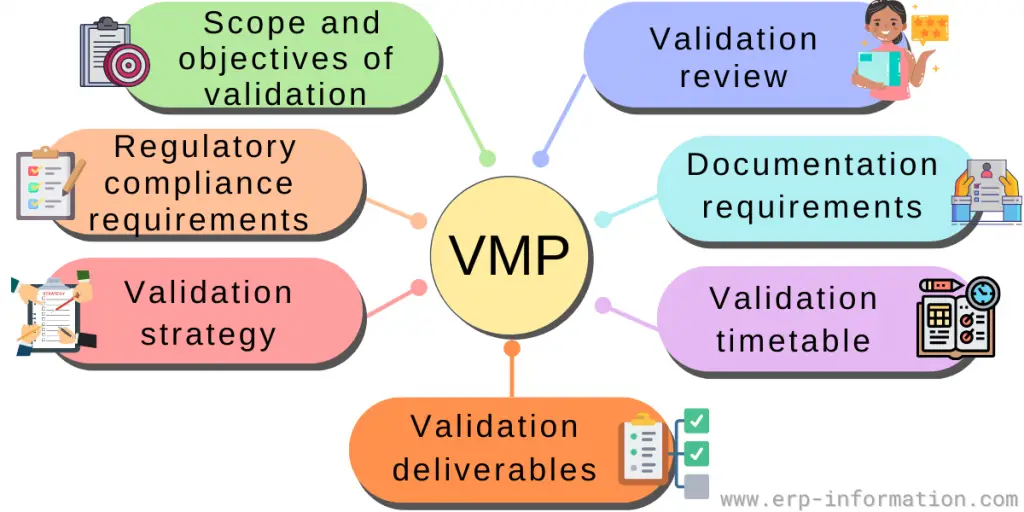
What is Validation Master Plan? (Template, Examples)
Documents Include Placeholder Marks For All.
Web Preview Validation Master Plan Template.
Web (Validation Master Plan) Or Lower Tier Documentation Alone May Cover The Qualification Of Materials.
In These Circumstances It Is Only The Bold And Confident That.
Related Post:
